Safety and early outcomes using a corticosteroid-avoidance immunosuppression protocol in pediatric heart transplant recipients
- PMID: 20061164
- PMCID: PMC4248357
- DOI: 10.1016/j.healun.2009.11.601
Safety and early outcomes using a corticosteroid-avoidance immunosuppression protocol in pediatric heart transplant recipients
Abstract
Background: Long-term oral corticosteroids have been a mainstay of maintenance immunosuppression in pediatric heart transplantation. In this study, we report early clinical outcomes in a cohort of pediatric heart transplant recipients managed using a steroid-avoidance protocol.
Methods: Of the 70 patients who underwent heart transplantation during the study period, 55 eligible recipients, including 49 non-sensitized and 6 sensitized (all 55 with negative crossmatch) patients, entered a steroid-avoidance immunosuppression protocol consisting of thymoglobin induction followed by a 2-drug, tacrolimus-based, corticosteroid-free regimen. The primary outcome variable was freedom from moderate rejection (International Society for Heart and Lung Transplantation [ISHLT] Grade 2R/3A or antibody-mediated rejection).
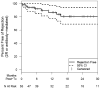
Results: The median age at transplant was 7.1 years (range 2 weeks to 22 years) and median follow-up was 19 months (range 2 to 46 months). Fifty patients survived to discharge after transplantation. Of these patients, 2 (4%) were discharged on steroids and 8 (16%) started on maintenance steroids at follow-up. Rejection was diagnosed in 8 patients (Grade 2R cellular rejection in 3 and antibody-mediated rejection in 5). Freedom from rejection was 92% at 6 months (95% confidence interval [CI] 80% to 97%) and 87% at 1 year (CI 73% to 94%). Post-transplant survival was 91% at 6 months (CI 79% to 96%) and 88% at 12 and 24 months (CI 75% to 95%). There was 1 death due to rejection (antibody-mediated) 8 months after transplantation.
Conclusions: An immunosuppression protocol consisting of induction followed by corticosteroid avoidance appears to achieve acceptable rejection rates during the first year post-transplant in pediatric heart transplant recipients.
Copyright (c) 2010 International Society for Heart and Lung Transplantation. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Early outcomes for low-risk pediatric heart transplant recipients and steroid avoidance: A multicenter cohort study (Clinical Trials in Organ Transplantation in Children - CTOTC-04).J Heart Lung Transplant. 2019 Sep;38(9):972-981. doi: 10.1016/j.healun.2019.06.006. Epub 2019 Jun 20. J Heart Lung Transplant. 2019. PMID: 31324444 Free PMC article.
-
New-onset diabetes mellitus in pediatric thoracic organ recipients receiving tacrolimus-based immunosuppression.J Heart Lung Transplant. 1997 Mar;16(3):275-82. J Heart Lung Transplant. 1997. PMID: 9087870
-
The effects of HLA mismatching and immunosuppressive therapy on early rejection outcome in pediatric heart transplant recipients.J Heart Lung Transplant. 1998 Dec;17(12):1195-200. J Heart Lung Transplant. 1998. PMID: 9883760
-
Tacrolimus: a further update of its use in the management of organ transplantation.Drugs. 2003;63(12):1247-97. doi: 10.2165/00003495-200363120-00006. Drugs. 2003. PMID: 12790696 Review.
-
Prevention and treatment of severe hemodynamic compromise in pediatric heart transplant patients.Paediatr Drugs. 2002;4(11):705-15. doi: 10.2165/00128072-200204110-00002. Paediatr Drugs. 2002. PMID: 12390042 Review.
Cited by
-
Early outcomes for low-risk pediatric heart transplant recipients and steroid avoidance: A multicenter cohort study (Clinical Trials in Organ Transplantation in Children - CTOTC-04).J Heart Lung Transplant. 2019 Sep;38(9):972-981. doi: 10.1016/j.healun.2019.06.006. Epub 2019 Jun 20. J Heart Lung Transplant. 2019. PMID: 31324444 Free PMC article.
-
Cardiac transplantation in children.BJA Educ. 2019 Apr;19(4):105-112. doi: 10.1016/j.bjae.2019.01.003. Epub 2019 Feb 10. BJA Educ. 2019. PMID: 33456878 Free PMC article. Review. No abstract available.
-
Clinical pharmacokinetics and pharmacodynamics of prednisolone and prednisone in solid organ transplantation.Clin Pharmacokinet. 2012 Nov;51(11):711-41. doi: 10.1007/s40262-012-0007-8. Clin Pharmacokinet. 2012. PMID: 23018468 Review.
-
A Proposal for Early Dosing Regimens in Heart Transplant Patients Receiving Thymoglobulin and Calcineurin Inhibition.Transplant Direct. 2016 May 20;2(6):e81. doi: 10.1097/TXD.0000000000000594. eCollection 2016 Jun. Transplant Direct. 2016. PMID: 27500271 Free PMC article. Review.
-
Immunosuppression therapy for pediatric heart transplantation.Curr Treat Options Cardiovasc Med. 2010 Oct;12(5):489-502. doi: 10.1007/s11936-010-0085-6. Curr Treat Options Cardiovasc Med. 2010. PMID: 20842569
References
-
- Canter CE, Moorhead S, Saffitz JE, Huddleston CB, Spray TL. Steroid withdrawal in the pediatric heart transplant recipient initially treated with triple immunosuppression. J Heart Lung Transplant. 1994;13:74–9. - PubMed
-
- Webber SA. 15 years of pediatric heart transplantation at the University of Pittsburgh: lessons learned and future prospects. Pediatr Transplant. 1997;1:8–21. - PubMed
-
- Boucek MM, Aurora P, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: tenth official pediatric heart transplantation report--2007. J Heart Lung Transplant. 2007;26:796–807. - PubMed
-
- Chinnock RE, Baum MF, Larsen R, Bailey L. Rejection management and long-term surveillance of the pediatric heart transplant recipient: the Loma Linda experience. J Heart Lung Transplant. 1993;12:S255–64. - PubMed
-
- Smith RR, Wray J, Khaghani A, Yacoub M. Ten year survival after paediatric heart transplantation: a single centre experience. Eur J Cardiothorac Surg. 2005;27:790–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical