The redox status of cystinotic fibroblasts
- PMID: 20061170
- PMCID: PMC2839033
- DOI: 10.1016/j.ymgme.2009.12.010
The redox status of cystinotic fibroblasts
Abstract
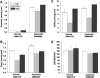
A key unresolved question in the pathogenesis of phenotype development in nephropathic cystinosis is whether intralysosomal cystine, the hallmark of this lethal inborn error of metabolism, alters cytoplasmic redox potential. Variable findings on this issue have been reported. This study of fetal and non-fetal skin and lung-derived cystinotic fibroblasts compared to origin and age-matched normal control fibroblasts reveals that cystinotic cells do not exhibit redox perturbations. We find that the steady-state redox status as assessed by the [GSH]/[GSSG] ratio, an indicator of the intracellular redox poise, is unchanged in cystinotic cells. Furthermore, the dependence of the intracellular GSH and cysteine pool sizes and the [GSH]/[GSSG] ratio are similarly dependent on the two major sources of cysteine, i.e. the transsulfuration pathway and the plasma membrane cystine transporter, xc(-), in both cystinotic and control cells, and the presence of lysosomal cystine has no measurable effect on the redox status of these cells. Hence, mechanisms other than cytosolic redox perturbations are involved in the etiology of nephropathic cystinosis.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
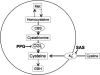
 CGL) preventing cysteine synthesis from methionine (Met) via the transsulfuration pathway. Sulfasalazine (SAS) inhibits the transmembrane cystine transporter xc-. Both inhibitors can decrease the intracellular cysteine concentration and thus cause a decrease in intracellular GSH concentration. CBS denotes cystathionine
CGL) preventing cysteine synthesis from methionine (Met) via the transsulfuration pathway. Sulfasalazine (SAS) inhibits the transmembrane cystine transporter xc-. Both inhibitors can decrease the intracellular cysteine concentration and thus cause a decrease in intracellular GSH concentration. CBS denotes cystathionine  -synthase, the first enzyme or transsulfuration pathway.
-synthase, the first enzyme or transsulfuration pathway.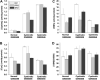
Similar articles
-
Altered status of glutathione and its metabolites in cystinotic cells.Nephrol Dial Transplant. 2005 Sep;20(9):1828-32. doi: 10.1093/ndt/gfh932. Epub 2005 Jun 14. Nephrol Dial Transplant. 2005. PMID: 15956064
-
Cysteamine restores glutathione redox status in cultured cystinotic proximal tubular epithelial cells.Biochim Biophys Acta. 2011 Jun;1812(6):643-51. doi: 10.1016/j.bbadis.2011.02.010. Epub 2011 Feb 28. Biochim Biophys Acta. 2011. PMID: 21371554
-
Lysosomal cystine storage augments apoptosis in cultured human fibroblasts and renal tubular epithelial cells.J Am Soc Nephrol. 2002 Dec;13(12):2878-87. doi: 10.1097/01.asn.0000036867.49866.59. J Am Soc Nephrol. 2002. PMID: 12444206
-
Potential role of apoptosis in development of the cystinotic phenotype.Pediatr Nephrol. 2005 Apr;20(4):441-6. doi: 10.1007/s00467-004-1712-9. Epub 2004 Dec 28. Pediatr Nephrol. 2005. PMID: 15622500 Review.
-
Redefining oxidative stress.Antioxid Redox Signal. 2006 Sep-Oct;8(9-10):1865-79. doi: 10.1089/ars.2006.8.1865. Antioxid Redox Signal. 2006. PMID: 16987039 Review.
Cited by
-
Gamma-glutamylcysteine synthetase and tryparedoxin 1 exert high control on the antioxidant system in Trypanosoma cruzi contributing to drug resistance and infectivity.Redox Biol. 2019 Sep;26:101231. doi: 10.1016/j.redox.2019.101231. Epub 2019 May 28. Redox Biol. 2019. PMID: 31203195 Free PMC article.
-
Programmed Cell Death in Cystinosis.Cells. 2022 Feb 15;11(4):670. doi: 10.3390/cells11040670. Cells. 2022. PMID: 35203319 Free PMC article. Review.
-
A deletion in the Ctns gene causes renal tubular dysfunction and cystine accumulation in LEA/Tohm rats.Mamm Genome. 2019 Feb;30(1-2):23-33. doi: 10.1007/s00335-018-9790-3. Epub 2018 Dec 27. Mamm Genome. 2019. PMID: 30591971 Free PMC article.
-
N-acetyl-cysteine is associated to renal function improvement in patients with nephropathic cystinosis.Pediatr Nephrol. 2014 Jun;29(6):1097-102. doi: 10.1007/s00467-013-2705-3. Epub 2013 Dec 11. Pediatr Nephrol. 2014. PMID: 24326786 Clinical Trial.
-
Biosynthesis and Reactivity of Cysteine Persulfides in Signaling.J Am Chem Soc. 2016 Jan 13;138(1):289-99. doi: 10.1021/jacs.5b10494. Epub 2015 Dec 28. J Am Chem Soc. 2016. PMID: 26667407 Free PMC article.
References
-
- Gahl WA, Thoene J, Schneider J. Cystinosis: A disorder of lysosomal membrane transport. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th edition McGraw Hill; 2001. pp. 5085–5108.
-
- Town M, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van't Hoff W, Antignac C. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nature Genet. 1998;18:319–324. - PubMed
-
- Oshima R, Rhead W, Thoene J, Schneider J. Cystine Metabolism in Human Fibroblasts. J. Biol. Chem. 1976;251:4287–4293. - PubMed
-
- Patrick A. Deficiences of -SH-dependent enzymes in cystinosis. J.Clin.Path. 1965;28:427–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

