Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis
- PMID: 20061183
- PMCID: PMC4617666
- DOI: 10.1016/S1474-4422(10)70002-8
Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis
Abstract
Background: Idiopathic Parkinson's disease can present with symptoms similar to those of multiple system atrophy or progressive supranuclear palsy. We aimed to assess whether metabolic brain imaging combined with spatial covariance analysis could accurately discriminate patients with parkinsonism who had different underlying disorders.
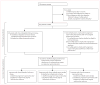
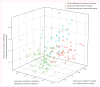
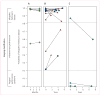
Methods: Between January, 1998, and December, 2006, patients from the New York area who had parkinsonian features but uncertain clinical diagnosis had fluorine-18-labelled-fluorodeoxyglucose-PET at The Feinstein Institute for Medical Research. We developed an automated image-based classification procedure to differentiate individual patients with idiopathic Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. For each patient, the likelihood of having each of the three diseases was calculated by use of multiple disease-related patterns with logistic regression and leave-one-out cross-validation. Each patient was classified according to criteria defined by receiver-operating-characteristic analysis. After imaging, patients were assessed by blinded movement disorders specialists for a mean of 2.6 years before a final clinical diagnosis was made. The accuracy of the initial image-based classification was assessed by comparison with the final clinical diagnosis.
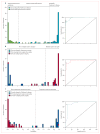
Findings: 167 patients were assessed. Image-based classification for idiopathic Parkinson's disease had 84% sensitivity, 97% specificity, 98% positive predictive value (PPV), and 82% negative predictive value (NPV). Imaging classifications were also accurate for multiple system atrophy (85% sensitivity, 96% specificity, 97% PPV, and 83% NPV) and progressive supranuclear palsy (88% sensitivity, 94% specificity, 91% PPV, and 92% NPV).
Interpretation: Automated image-based classification has high specificity in distinguishing between parkinsonian disorders and could help in selecting treatment for early-stage patients and identifying participants for clinical trials.
Funding: National Institutes of Health and General Clinical Research Center at The Feinstein Institute for Medical Research.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
DE is co-inventor of US patents 5 632 276 (filed Jan 27, 1995, granted May 27, 1997) and 5 873 823 (filed Sept 4, 1996, granted Feb 23, 1999) for the use of spatial patterns for the diagnosis of brain disease. DE has no financial conflicts of interest. All other authors have no conflicts of interest.
Figures

References
-
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 2001;57:S34–38. - PubMed
-
- Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–70. - PubMed
-
- Shih LC, Tarsy D. Deep brain stimulation for the treatment of atypical parkinsonism. Mov Disord. 2007;22:2149–55. - PubMed
-
- Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. - PubMed
-
- Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287:1653–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

