Distinct roles of RECQ1 in the maintenance of genomic stability
- PMID: 20061189
- PMCID: PMC2827648
- DOI: 10.1016/j.dnarep.2009.12.010
Distinct roles of RECQ1 in the maintenance of genomic stability
Abstract
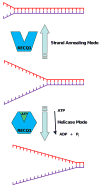
Five human RecQ helicases (WRN, BLM, RECQ4, RECQ5, RECQ1) exist in humans. Of these, three are genetically linked to diseases of premature aging and/or cancer. Neither RECQ1 nor RECQ5 has yet been implicated in a human disease. However, cellular studies and genetic analyses of model organisms indicate that RECQ1 (and RECQ5) play an important role in the maintenance of genomic stability. Biochemical studies of purified RECQ1 protein demonstrate that the enzyme catalyzes DNA unwinding and strand annealing, and these activities are likely to be important for its role in DNA repair. RECQ1 also physically and functionally interacts with proteins involved in genetic recombination. In this review, we will summarize our current knowledge of RECQ1 roles in cellular nucleic acid metabolism and propose avenues of investigation for future studies.
(c) 2009 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Catalytic strand separation by RECQ1 is required for RPA-mediated response to replication stress.Curr Biol. 2015 Nov 2;25(21):2830-2838. doi: 10.1016/j.cub.2015.09.026. Epub 2015 Oct 8. Curr Biol. 2015. PMID: 26455304 Free PMC article.
-
RECQ1 possesses DNA branch migration activity.J Biol Chem. 2008 Jul 18;283(29):20231-42. doi: 10.1074/jbc.M801582200. Epub 2008 May 21. J Biol Chem. 2008. PMID: 18495662 Free PMC article.
-
Conserved helicase domain of human RecQ4 is required for strand annealing-independent DNA unwinding.DNA Repair (Amst). 2010 Jul 1;9(7):796-804. doi: 10.1016/j.dnarep.2010.04.003. Epub 2010 May 6. DNA Repair (Amst). 2010. PMID: 20451470 Free PMC article.
-
Roles of Werner syndrome protein in protection of genome integrity.DNA Repair (Amst). 2010 Mar 2;9(3):331-44. doi: 10.1016/j.dnarep.2009.12.011. Epub 2010 Jan 13. DNA Repair (Amst). 2010. PMID: 20075015 Free PMC article. Review.
-
Mutations in conserved functional domains of human RecQ helicases are associated with diseases and cancer: A review.Biophys Chem. 2020 Oct;265:106433. doi: 10.1016/j.bpc.2020.106433. Epub 2020 Jul 16. Biophys Chem. 2020. PMID: 32702531 Review.
Cited by
-
Site-directed mutants of human RECQ1 reveal functional importance of the zinc binding domain.Mutat Res. 2016 Aug;790:8-18. doi: 10.1016/j.mrfmmm.2016.05.005. Epub 2016 May 17. Mutat Res. 2016. PMID: 27248010 Free PMC article.
-
Superfamily 2 helicases.Front Biosci (Landmark Ed). 2012 Jun 1;17(6):2070-88. doi: 10.2741/4038. Front Biosci (Landmark Ed). 2012. PMID: 22652765 Free PMC article. Review.
-
Remodeling and Control of Homologous Recombination by DNA Helicases and Translocases that Target Recombinases and Synapsis.Genes (Basel). 2016 Aug 19;7(8):52. doi: 10.3390/genes7080052. Genes (Basel). 2016. PMID: 27548227 Free PMC article. Review.
-
RECQL: a new breast cancer susceptibility gene.Cell Cycle. 2015;14(22):3540-3. doi: 10.1080/15384101.2015.1066539. Cell Cycle. 2015. PMID: 26125302 Free PMC article.
-
Both OsRecQ1 and OsRDR1 are required for the production of small RNA in response to DNA-damage in rice.PLoS One. 2013;8(1):e55252. doi: 10.1371/journal.pone.0055252. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23383126 Free PMC article.
References
-
- Puranam KL, Blackshear PJ. Cloning and characterization of RECQL, a potential human homologue of the Escherichia coli DNA helicase RecQ. J Biol Chem. 1994;269:29838–29845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

