The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study
- PMID: 20061360
- PMCID: PMC2803743
- DOI: 10.1136/bmj.b5444
The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study
Abstract
Objectives: To investigate potential determinants of severe hypoglycaemia, including baseline characteristics, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial and the association of severe hypoglycaemia with levels of glycated haemoglobin (haemoglobin A(1C)) achieved during therapy.
Design: Post hoc epidemiological analysis of a double 2x2 factorial, randomised, controlled trial.
Setting: Diabetes clinics, research clinics, and primary care clinics.
Participants: 10 209 of the 10 251 participants enrolled in the ACCORD study with type 2 diabetes, a haemoglobin A(1C) concentration of 7.5% or more during screening, and aged 40-79 years with established cardiovascular disease or 55-79 years with evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or two or more additional risk factors for cardiovascular disease (dyslipidaemia, hypertension, current smoker, or obese). Interventions Intensive (haemoglobin A(1C) <6.0%) or standard (haemoglobin A(1C) 7.0-7.9%) glucose control.
Main outcome measures: Severe hypoglycaemia was defined as episodes of "low blood glucose" requiring the assistance of another person and documentation of either a plasma glucose less than 2.8 mmol/l (<50 mg/dl) or symptoms that promptly resolved with oral carbohydrate, intravenous glucose, or glucagon.
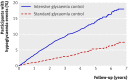
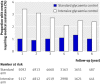
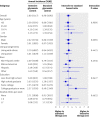
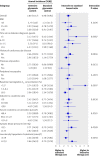
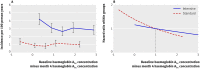
Results: The annual incidence of hypoglycaemia was 3.14% in the intensive treatment group and 1.03% in the standard glycaemia group. We found significantly increased risks for hypoglycaemia among women (P=0.0300), African-Americans (P<0.0001 compared with non-Hispanic whites), those with less than a high school education (P<0.0500 compared with college graduates), aged participants (P<0.0001 per 1 year increase), and those who used insulin at trial entry (P<0.0001). For every 1% unit decline in the haemoglobin A(1C) concentration from baseline to 4 month visit, there was a 28% (95% CI 19% to 37%) and 14% (4% to 23%) reduced risk of hypoglycaemia requiring medical assistance in the standard and intensive groups, respectively. In both treatment groups, the risk of hypoglycaemia requiring medical assistance increased with each 1% unit increment in the average updated haemoglobin A(1C) concentration (standard arm: hazard ratio 1.76, 95% CI 1.50 to 2.06; intensive arm: hazard ratio 1.15, 95% CI 1.02 to 1.21).
Conclusions: A greater drop in haemoglobin A(1C) concentration from baseline to the 4 month visit was not associated with an increased risk for hypoglycaemia. Patients with poorer glycaemic control had a greater risk of hypoglycaemia, irrespective of treatment group. Identification of baseline subgroups with increased risk for severe hypoglycaemia can provide guidance to clinicians attempting to modify patient therapy on the basis of individual risk.
Trial registration: ClinicalTrials.gov number NCT00000620.
Conflict of interest statement
Competing interests: MEM has received honorariums for consulting from Hoffman-LaRoche. HCG has received research grants and honorariums for consulting and speaking from the following companies that manufacture glucose lowering medications: Sanofi-Aventis, GlaxoSmithKline, and Merck. He has also received honorariums for consulting and/or speaking from NovoNordisk, Lilly, Roche, BristolMyersSquibb, and AstraZeneca. HCG holds the Population Health Institute chair in diabetes research sponsored by Aventis. ERS has served as a consultant for Merck and has reviewed applications for the Pfizer Visiting Professorship Program. RMB and RMC have served as principal investigator or coinvestigator for industry sponsored clinical trials research. All honorariums, speaking fees, consulting fees, and research and educational support are paid directly to the non-profit International Diabetes Center, of which RMB and RMC are salaried employees. JCE is a member of Merck and Sanofi-Aventis speaker’s bureau and holds a clinical grant funded by Merck. RDC has received honorariums for consulting and speaking from the following companies that manufacture glucose lowering medications: Sanofi-Aventis, GlaxoSmithKline, and Merck. He has also received honorariums for consulting and/or speaking from Pfizer and has served as a consultant for NovoNordisk. GD has served as an investigator, speaker, and occasional consultant for Sanofi-Aventis and Merck, and as an investigator for NovoNordisk, Eli Lilly/Amylin, Mannkind, Pfizer, Boeringer Ingelheim, Novartis, GlaxoSmithKline, and BristolMyersSquibb. JFL has received honorariums from the American Academy of Physician Assistants, Amylin, AstraZeneca, NovoNordisk, and Smith’s Medical, has been a consultant for AstraZeneca, and has received grants or research support from the following companies: Amylin; Hoffman-LaRoche; Medtronic; NovoNordisk; Novartis; Osiris; Pfizer; National Institutes for Heath/National Heart, Lung, and Blood Institute; Tranistion Therapeutics; and Tolerx. AS has received consulting honorariums from Pfizer, Novartis, and Medtronic, speaking fees from Pfizer, and was a principal investigator of a clinical trial sponsored by Sanofi-Aventis. SG has been a consultant for Merck and Sanofi-Aventis, and owns stock in Johnson and Johnson and in Novartis (<$5000 each). The remaining authors have no competing interests.
Figures


References
-
- Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 2003;26:1485-9. - PubMed
-
- Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 2002;45:937-48. - PubMed
-
- DCCT Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med 1991;90:450-9. - PubMed
-
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al for the ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-72. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
