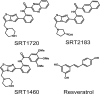
SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1
- PMID: 20061378
- PMCID: PMC2832984
- DOI: 10.1074/jbc.M109.088682
SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1
Abstract
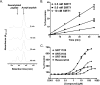
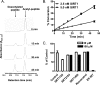
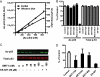
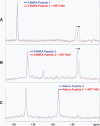
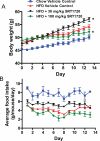
Sirtuins catalyze NAD(+)-dependent protein deacetylation and are critical regulators of transcription, apoptosis, metabolism, and aging. There are seven human sirtuins (SIRT1-7), and SIRT1 has been implicated as a key mediator of the pathways downstream of calorie restriction that have been shown to delay the onset and reduce the incidence of age-related diseases such as type 2 diabetes. Increasing SIRT1 activity, either by transgenic overexpression of the Sirt1 gene in mice or by pharmacological activation by small molecule activators resveratrol and SRT1720, has shown beneficial effects in rodent models of type 2 diabetes, indicating that SIRT1 may represent an attractive therapeutic target. Herein, we have assessed purported SIRT1 activators by employing biochemical assays utilizing native substrates, including a p53-derived peptide substrate lacking a fluorophore as well as the purified native full-length protein substrates p53 and acetyl-CoA synthetase1. SRT1720, its structurally related compounds SRT2183 and SRT1460, and resveratrol do not lead to apparent activation of SIRT1 with native peptide or full-length protein substrates, whereas they do activate SIRT1 with peptide substrate containing a covalently attached fluorophore. Employing NMR, surface plasmon resonance, and isothermal calorimetry techniques, we provide evidence that these compounds directly interact with fluorophore-containing peptide substrates. Furthermore, we demonstrate that SRT1720 neither lowers plasma glucose nor improves mitochondrial capacity in mice fed a high fat diet. SRT1720, SRT2183, SRT1460, and resveratrol exhibit multiple off-target activities against receptors, enzymes, transporters, and ion channels. Taken together, we conclude that SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1.
Figures
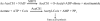


References
-
- Moynihan K. A., Grimm A. A., Plueger M. M., Bernal-Mizrachi E., Ford E., Cras-Méneur C., Permutt M. A., Imai S. (2005) Cell Metab. 2, 105–117 - PubMed
-
- Starai V. J., Celic I., Cole R. N., Boeke J. D., Escalante-Semerena J. C. (2002) Science 298, 2390–2392 - PubMed
-
- Liang F., Kume S., Koya D. (2009) Nat. Rev. Endocrinol. 5, 367–373 - PubMed
-
- Sauve A. A., Wolberger C., Schramm V. L., Boeke J. D. (2006) Annu. Rev. Biochem. 75, 435–465 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

