Pro-hormone secretogranin II regulates dense core secretory granule biogenesis in catecholaminergic cells
- PMID: 20061385
- PMCID: PMC2843166
- DOI: 10.1074/jbc.M109.064196
Pro-hormone secretogranin II regulates dense core secretory granule biogenesis in catecholaminergic cells
Abstract
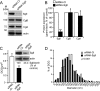
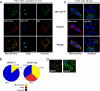
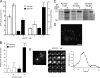
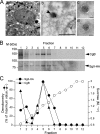
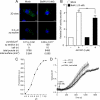
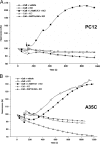
Processes underlying the formation of dense core secretory granules (DCGs) of neuroendocrine cells are poorly understood. Here, we present evidence that DCG biogenesis is dependent on the secretory protein secretogranin (Sg) II, a member of the granin family of pro-hormone cargo of DCGs in neuroendocrine cells. Depletion of SgII expression in PC12 cells leads to a decrease in both the number and size of DCGs and impairs DCG trafficking of other regulated hormones. Expression of SgII fusion proteins in a secretory-deficient PC12 variant rescues a regulated secretory pathway. SgII-containing dense core vesicles share morphological and physical properties with bona fide DCGs, are competent for regulated exocytosis, and maintain an acidic luminal pH through the V-type H(+)-translocating ATPase. The granulogenic activity of SgII requires a pH gradient along this secretory pathway. We conclude that SgII is a critical factor for the regulation of DCG biogenesis in neuroendocrine cells, mediating the formation of functional DCGs via its pH-dependent aggregation at the trans-Golgi network.
Figures


Similar articles
-
Sorting of the neuroendocrine secretory protein Secretogranin II into the regulated secretory pathway: role of N- and C-terminal alpha-helical domains.J Biol Chem. 2008 Apr 25;283(17):11807-22. doi: 10.1074/jbc.M709832200. Epub 2008 Feb 25. J Biol Chem. 2008. PMID: 18299326 Free PMC article.
-
Rab3D is critical for secretory granule maturation in PC12 cells.PLoS One. 2013;8(3):e57321. doi: 10.1371/journal.pone.0057321. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23526941 Free PMC article.
-
Multiple sorting systems for secretory granules ensure the regulated secretion of peptide hormones.Traffic. 2013 Feb;14(2):205-18. doi: 10.1111/tra.12029. Epub 2012 Dec 7. Traffic. 2013. PMID: 23171199
-
Chromogranins A and B and secretogranin II: evolutionary and functional aspects.Acta Physiol (Oxf). 2008 Feb;192(2):309-24. doi: 10.1111/j.1748-1716.2007.01806.x. Epub 2007 Nov 15. Acta Physiol (Oxf). 2008. PMID: 18005393 Review.
-
Chromogranin A: a new proposal for trafficking, processing and induction of granule biogenesis.Regul Pept. 2010 Feb 25;160(1-3):153-9. doi: 10.1016/j.regpep.2009.12.007. Epub 2009 Dec 16. Regul Pept. 2010. PMID: 20006653 Free PMC article. Review.
Cited by
-
The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure.FASEB J. 2014 May;28(5):2120-33. doi: 10.1096/fj.13-239509. Epub 2014 Feb 4. FASEB J. 2014. PMID: 24497580 Free PMC article.
-
The BAR domain protein PICK1 controls vesicle number and size in adrenal chromaffin cells.J Neurosci. 2014 Aug 6;34(32):10688-700. doi: 10.1523/JNEUROSCI.5132-13.2014. J Neurosci. 2014. PMID: 25100601 Free PMC article.
-
Dense-core vesicle biogenesis and exocytosis in neurons lacking chromogranins A and B.J Neurochem. 2018 Feb;144(3):241-254. doi: 10.1111/jnc.14263. Epub 2017 Dec 27. J Neurochem. 2018. PMID: 29178418 Free PMC article.
-
A common genetic variant in the 3'-UTR of vacuolar H+-ATPase ATP6V0A1 creates a micro-RNA motif to alter chromogranin A processing and hypertension risk.Circ Cardiovasc Genet. 2011 Aug 1;4(4):381-9. doi: 10.1161/CIRCGENETICS.111.959767. Epub 2011 May 9. Circ Cardiovasc Genet. 2011. PMID: 21558123 Free PMC article.
-
Trafficking of hormones and trophic factors to secretory and extracellular vesicles: a historical perspective and new hypothesis.Extracell Vesicles Circ Nucl Acids. 2023;4(4):568-587. doi: 10.20517/evcna.2023.34. Epub 2023 Nov 9. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 38435713 Free PMC article.
References
-
- Taupenot L., Harper K. L., O'Connor D. T. (2003) N. Engl. J. Med. 348, 1134–1149 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

