The natural viral load profile of patients with pandemic 2009 influenza A(H1N1) and the effect of oseltamivir treatment
- PMID: 20061398
- PMCID: PMC7094292
- DOI: 10.1378/chest.09-3072
The natural viral load profile of patients with pandemic 2009 influenza A(H1N1) and the effect of oseltamivir treatment
Abstract
Background: The natural history of viral shedding from the upper respiratory tract of the new pandemic 2009 influenza A(H1N1) and the effect of oseltamivir treatment were uncertain.
Methods: A retrospective cohort study involving 145 consecutive patients with specimens positive by reverse transcriptase-polymerase chain reaction for the matrix and new H1 genes was conducted.
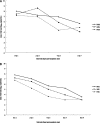
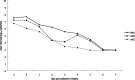
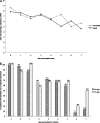
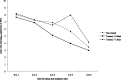
Results: The nontreated and oseltamivir-treated patients were comparable in their viral load at presentation, demography, and the presenting symptoms. No correlation was observed between viral load with age and number of symptoms. Viral load of nasopharyngeal aspirate (NPA) was significantly lower in treated than in nontreated patients at day 5 after symptom onset. When oseltamivir was initiated </= 2 days after symptom onset, a greater rate of viral load reduction in NPA of treated patients than that of nontreated patients was observed (-0.638 [95% CI, -0.809 to -0.466] vs -0.409 [95% CI, -0.663 to -0.185] log(10) copies/mL/d post-symptom onset), and the viral load was undetectable at day 6 after oseltamivir initiation, which was 1 day earlier than that of those whose treatment was initiated > 2 days of symptom onset. The viral load was inversely correlated with concomitant absolute lymphocyte count in nontreated patients (Pearson correlation coefficient [r] = -0.687, P = .001) and treated patients (Pearson r = -0.365, P < .001). Resolution of fever was 1.4 days later in nontreated than treated patients (P = .012)
Conclusions: The natural viral load profile was described. Oral oseltamivir suppresses viral load more effectively when given early in mild cases of pandemic 2009 influenza A(H1N1) infections.
Figures
Comment in
-
A real-time reverse transcriptase-polymer chain reaction to evaluate natural history of viral shedding in outpatient children and adolescents with pandemic 2009 influenza A(H1N1).Chest. 2010 Aug;138(2):456-7; author reply 457-8. doi: 10.1378/chest.10-0323. Chest. 2010. PMID: 20682541 No abstract available.
Similar articles
-
Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China.BMJ. 2010 Sep 28;341:c4779. doi: 10.1136/bmj.c4779. BMJ. 2010. PMID: 20876641 Free PMC article.
-
Effects of early oseltamivir therapy on viral shedding in 2009 pandemic influenza A (H1N1) virus infection.Clin Infect Dis. 2010 Apr 1;50(7):963-9. doi: 10.1086/651083. Clin Infect Dis. 2010. PMID: 20180701
-
Factors promoting the prolonged shedding of the pandemic (H1N1) 2009 influenza virus in patients treated with oseltamivir for 5 days.Influenza Other Respir Viruses. 2013 Sep;7(5):833-7. doi: 10.1111/irv.12065. Epub 2012 Dec 26. Influenza Other Respir Viruses. 2013. PMID: 23279949 Free PMC article.
-
The use of antiviral agents for the management of severe influenza.Crit Care Med. 2010 Apr;38(4 Suppl):e43-51. doi: 10.1097/CCM.0b013e3181c85229. Crit Care Med. 2010. PMID: 19935416 Review.
-
Oseltamivir in human avian influenza infection.J Antimicrob Chemother. 2010 Apr;65 Suppl 2(Suppl 2):ii25-ii33. doi: 10.1093/jac/dkq013. J Antimicrob Chemother. 2010. PMID: 20215132 Free PMC article. Review.
Cited by
-
Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial.BMJ. 2013 May 30;346:f3039. doi: 10.1136/bmj.f3039. BMJ. 2013. PMID: 23723457 Free PMC article. Clinical Trial.
-
Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic--the risk of aerosol generation during medical procedures.PLoS One. 2013;8(2):e56278. doi: 10.1371/journal.pone.0056278. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418548 Free PMC article.
-
The epidemiological and public health research response to 2009 pandemic influenza A(H1N1): experiences from Hong Kong.Influenza Other Respir Viruses. 2013 May;7(3):367-82. doi: 10.1111/j.1750-2659.2012.00420.x. Epub 2012 Aug 9. Influenza Other Respir Viruses. 2013. PMID: 22883352 Free PMC article. Review.
-
Controlled Human Infection Challenge Studies with RSV.Curr Top Microbiol Immunol. 2024;445:41-68. doi: 10.1007/82_2022_257. Curr Top Microbiol Immunol. 2024. PMID: 35704096 Review.
-
Comparative epidemiology of pandemic and seasonal influenza A in households.N Engl J Med. 2010 Jun 10;362(23):2175-2184. doi: 10.1056/NEJMoa0911530. N Engl J Med. 2010. PMID: 20558368 Free PMC article.
References
-
- World Health Organization Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Wkly Epidemiol Rec. 2009;84(21):185–189. - PubMed
-
- World Health Organization Statement to the press by WHO Director-General Dr Margaret Chan June 11 2009 World now at the start of 2009 influenza pandemic. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6... Accessed August 3, 2009.
-
- Hancock K, Veguilla V, Lu X. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945–1952. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

