Growth hormone deficiency after treatment of acromegaly: a randomized, placebo-controlled study of growth hormone replacement
- PMID: 20061426
- PMCID: PMC2840863
- DOI: 10.1210/jc.2009-1611
Growth hormone deficiency after treatment of acromegaly: a randomized, placebo-controlled study of growth hormone replacement
Abstract
Context: The effects of GH replacement therapy in patients who develop GH deficiency (GHD) after cure of acromegaly have not been established in a placebo-controlled study.
Objective: The objective of the study was to determine whether GH replacement improves body composition, cardiovascular risk markers and quality of life in patients with GHD and prior acromegaly.
Design: This was a 6-month, randomized, placebo-controlled study.
Setting: The study was conducted at a clinical translational science center.
Study participants: Participants included 30 subjects with prior acromegaly and current GHD.
Intervention: INTERVENTIONs included GH or placebo.
Main outcome measures: Body composition (dual-energy x-ray absorptiometry and cross-sectional computed tomography at L4), cardiovascular risk markers (high-sensitivity C-reactive protein (hsCRP), total, high-density lipoprotein and low-density lipoprotein cholesterol, fibrinogen, and carotid intimal-medial thickness), and quality of life were measured.
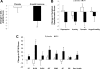
Results: The mean GH dose at 6 months was 0.58 +/- 0.26 mg/d. Total fat mass, visceral adipose tissue (-15.3 +/- 18.6 vs. 1.3 +/- 12.5%, P = 0.01), and total abdominal fat decreased, and fat-free mass increased, in the GH vs. placebo group. Mean hsCRP levels decreased, but there was no GH effect on other cardiovascular risk markers. There was no change in glycosylated hemoglobin or homeostasis model assessment insulin resistance index. Quality of life improved with GH. Side effects were minimal.
Conclusions: This is the first randomized, placebo-controlled study of the effects of GH replacement therapy on body composition and cardiovascular end points in patients who have developed GH deficiency after treatment for acromegaly, a disease complicated by metabolic and body composition alterations and increased cardiovascular risk. GH replacement decreased visceral adipose tissue, increased fat-free mass, decreased hsCRP, and improved quality of life in patients with GHD after cure of acromegaly, with minimal side effects and without an increase in insulin resistance.
Figures
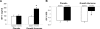

References
-
- Barreto-Filho JA, Alcântara MR, Salvatori R, Barreto MA, Sousa AC, Bastos V, Souza AH, Pereira RM, Clayton PE, Gill MS, Aguiar-Oliveira MH 2002 Familial isolated growth hormone deficiency is associated with increased systolic blood pressure, central obesity, and dyslipidemia. J Clin Endocrinol Metab 87:2018–2023 - PubMed
-
- Binnerts A, Deurenberg P, Swart GR, Wilson JH, Lamberts SW 1992 Body composition in growth hormone-deficient adults. Am J Clin Nutr 55:918–923 - PubMed
-
- De Boer H, Blok GJ, Voerman HJ, De Vries PM, van der Veen EA 1992 Body composition in adult growth hormone-deficient men, assessed by anthropometry and bioimpedance analysis. J Clin Endocrinol Metab 75:833–837 - PubMed
-
- Devin JK, Blevins Jr LS, Verity DK, Chen Q, Bloodworth Jr JR, Covington J, Vaughan DE 2007 Markedly impaired fibrinolytic balance contributes to cardiovascular risk in adults with growth hormone deficiency. J Clin Endocrinol Metab 92:3633–3639 - PubMed
-
- Gomez JM, Sahun M, Vila R, Domenech P, Catalina P, Soler J, Badimon L 2006 Peripheral fibrinolytic markers, soluble adhesion molecules, inflammatory cytokines and endothelial function in hypopituitary adults with growth hormone deficiency. Clin Endocrinol (Oxf) 64:632–639 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

