Identification and in vitro characterization of follicle stimulating hormone (FSH) receptor variants associated with abnormal ovarian response to FSH
- PMID: 20061434
- PMCID: PMC2840851
- DOI: 10.1210/jc.2009-1304
Identification and in vitro characterization of follicle stimulating hormone (FSH) receptor variants associated with abnormal ovarian response to FSH
Abstract
Context: FSH mediates cyclic follicle growth and development and is widely used for controlled ovarian stimulation in women undergoing infertility treatment. The ovarian response of women to FSH is variable, ranging from poor response to ovarian hyperstimulation.
Objective: We investigated whether genetic alterations of the FSH receptor (FSHR) contribute to this variability.
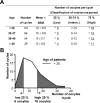
Design and patients: Our approach was to study women undergoing treatment with in vitro fertilization falling into the edges of the normal distribution of ovarian response to FSH, with respect to age.
Setting: We conducted the study at the Yale Fertility Clinic.
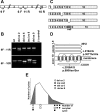
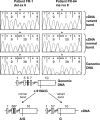
Methods: We extracted RNA from cumulus cells surrounding the oocytes of women undergoing in vitro fertilization and analyzed the FSHR mRNA by RT-PCR and sequencing.
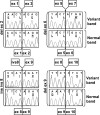
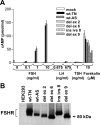
Results: We identified four abnormal FSHR splicing products (three exon deletions and one intron insertion) in the FSHR mRNA in 37% (13 of 35) of women tested. All alterations affected the extracellular ligand-binding portion of the receptor without causing a frameshift. When transfected in HEK293T cells, all four splicing variants showed markedly decreased cAMP activation compared to controls. Untransfected cells showed no response to FSH, whereas all the cell lines showed normal cAMP activation when treated with forskolin, a nonreceptor-mediated cAMP stimulant. None of the normal or mutant forms showed any response to LH or TSH.
Conclusions: Our findings strongly indicate FSHR variants as being an intrinsic genetic cause of some forms of infertility and identify a need for functional characterization of these variants and the investigation of more individualized ovarian stimulation protocols.
Figures
References
-
- Huhtaniemi IT, Themmen AP 2005 Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine 26:207–217 - PubMed
-
- Camp TA, Rahal JO, Mayo KE 1991 Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol Endocrinol 5:1405–1417 - PubMed
-
- Sprengel R, Braun T, Nikolics K, Segaloff DL, Seeburg PH 1990 The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol Endocrinol 4:525–530 - PubMed
-
- Kelton CA, Cheng SV, Nugent NP, Schweickhardt RL, Rosenthal JL, Overton SA, Wands GD, Kuzeja JB, Luchette CA, Chappel SC 1992 The cloning of the human follicle stimulating hormone receptor and its expression in COS-7, CHO, and Y-1 cells. Mol Cell Endocrinol 89:141–151 - PubMed
-
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M 2006 FSH directly regulates bone mass. Cell 125:247–260 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

