Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling
- PMID: 20061450
- PMCID: PMC2832271
- DOI: 10.1104/pp.109.148007
Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling
Abstract
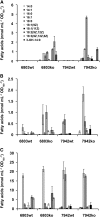
In cyanobacteria fatty acids destined for lipid synthesis can be synthesized de novo, but also exogenous free fatty acids from the culture medium can be directly incorporated into lipids. Activation of exogenous fatty acids is likely required prior to their utilization. To identify the enzymatic activity responsible for activation we cloned candidate genes from Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942 and identified the encoded proteins as acyl-acyl carrier protein synthetases (Aas). The enzymes catalyze the ATP-dependent esterification of fatty acids to the thiol of acyl carrier protein. The two protein sequences are only distantly related to known prokaryotic Aas proteins but they display strong similarity to sequences that can be found in almost all organisms that perform oxygenic photosynthesis. To investigate the biological role of Aas activity in cyanobacteria, aas knockout mutants were generated in the background of Synechocystis sp. PCC 6803 and S. elongatus PCC 7942. The mutant strains showed two phenotypes characterized by the inability to utilize exogenous fatty acids and by the secretion of endogenous fatty acids into the culture medium. The analyses of extracellular and intracellular fatty acid profiles of aas mutant strains as well as labeling experiments indicated that the detected free fatty acids are released from membrane lipids. The data suggest a considerable turnover of lipid molecules and a role for Aas activity in recycling the released fatty acids. In this model, lipid degradation represents a third supply of fatty acids for lipid synthesis in cyanobacteria.
Figures






Similar articles
-
Essential Role of Acyl-ACP Synthetase in Acclimation of the Cyanobacterium Synechococcus elongatus Strain PCC 7942 to High-Light Conditions.Plant Cell Physiol. 2015 Aug;56(8):1608-15. doi: 10.1093/pcp/pcv086. Epub 2015 Jun 10. Plant Cell Physiol. 2015. PMID: 26063393
-
Enzymatic and physiological characterization of fatty acid activation in Synechocystis sp. PCC6803.J Basic Microbiol. 2013 Oct;53(10):848-55. doi: 10.1002/jobm.201200228. Epub 2013 Feb 18. J Basic Microbiol. 2013. PMID: 23417914
-
A simple method for isolation and construction of markerless cyanobacterial mutants defective in acyl-acyl carrier protein synthetase.Appl Microbiol Biotechnol. 2016 Dec;100(23):10107-10113. doi: 10.1007/s00253-016-7850-8. Epub 2016 Oct 4. Appl Microbiol Biotechnol. 2016. PMID: 27704180 Free PMC article.
-
Incorporation, fate, and turnover of free fatty acids in cyanobacteria.FEMS Microbiol Rev. 2023 Mar 10;47(2):fuad015. doi: 10.1093/femsre/fuad015. FEMS Microbiol Rev. 2023. PMID: 37061785 Free PMC article. Review.
-
Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation.Biochim Biophys Acta. 2007 Mar;1771(3):286-98. doi: 10.1016/j.bbalip.2006.05.003. Epub 2006 May 16. Biochim Biophys Acta. 2007. PMID: 16798075 Review.
Cited by
-
Lauric Acid Production in a Glycogen-Less Strain of Synechococcus sp. PCC 7002.Front Bioeng Biotechnol. 2015 Apr 24;3:48. doi: 10.3389/fbioe.2015.00048. eCollection 2015. Front Bioeng Biotechnol. 2015. PMID: 25964950 Free PMC article.
-
Specific Incorporation of Polyunsaturated Fatty Acids into the sn-2 Position of Phosphatidylglycerol Accelerates Photodamage to Photosystem II under Strong Light.Int J Mol Sci. 2021 Sep 28;22(19):10432. doi: 10.3390/ijms221910432. Int J Mol Sci. 2021. PMID: 34638772 Free PMC article.
-
Biodiesel Production from the Marine Alga Nannochloropsis oceanica Grown on Yeast Wastewater and the Effect on Its Biochemical Composition and Gene Expression.Plants (Basel). 2023 Aug 8;12(16):2898. doi: 10.3390/plants12162898. Plants (Basel). 2023. PMID: 37631110 Free PMC article.
-
Hydrocarbon Desaturation in Cyanobacterial Thylakoid Membranes Is Linked With Acclimation to Suboptimal Growth Temperatures.Front Microbiol. 2021 Nov 26;12:781864. doi: 10.3389/fmicb.2021.781864. eCollection 2021. Front Microbiol. 2021. PMID: 34899663 Free PMC article.
-
FadD is required for utilization of endogenous fatty acids released from membrane lipids.J Bacteriol. 2011 Nov;193(22):6295-304. doi: 10.1128/JB.05450-11. Epub 2011 Sep 16. J Bacteriol. 2011. PMID: 21926226 Free PMC article.
References
-
- Babbitt PC, Kenyon GL, Martin BM, Charest H, Slyvestre M, Scholten JD, Chang KH, Liang PH, Dunaway-Mariano D. (1992) Ancestry of the 4-chlorobenzoate dehalogenase: analysis of amino acid sequence identities among families of acyl:adenyl ligases, enoyl-CoA hydratases/isomerases, and acyl-CoA thioesterases. Biochemistry 31: 5594–5604 - PubMed
-
- Bligh EG, Dyer WJ. (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 - PubMed
-
- Christie WW. (1982) A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J Lipid Res 23: 1072–1075 - PubMed
-
- Felsenstein J. (1989) PHYLIP—Phylogeny Interference Package (Version 3.2). Cladistics 5: 164–166
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

