The chemoreceptor dimer is the unit of conformational coupling and transmembrane signaling
- PMID: 20061469
- PMCID: PMC2820847
- DOI: 10.1128/JB.01391-09
The chemoreceptor dimer is the unit of conformational coupling and transmembrane signaling
Abstract
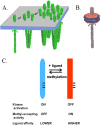
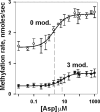
Transmembrane chemoreceptors are central components in bacterial chemotaxis. Receptors couple ligand binding and adaptational modification to receptor conformation in processes that create transmembrane signaling. Homodimers, the fundamental receptor structural units, associate in trimers and localize in patches of thousands. To what degree do conformational coupling and transmembrane signaling require higher-order interactions among dimers? To what degree are they altered by such interactions? To what degree are they inherent features of homodimers? We addressed these questions using nanodiscs to create membrane environments in which receptor dimers had few or no potential interaction partners. Receptors with many, few, or no interaction partners were tested for conformational changes and transmembrane signaling in response to ligand occupancy and adaptational modification. Conformation was assayed by measuring initial rates of receptor methylation, a parameter independent of receptor-receptor interactions. Coupling of ligand occupancy and adaptational modification to receptor conformation and thus to transmembrane signaling occurred with essentially the same sensitivity and magnitude in isolated dimers as for dimers with many neighbors. Thus, we conclude that the chemoreceptor dimer is the fundamental unit of conformational coupling and transmembrane signaling. This implies that in signaling complexes, coupling and transmembrane signaling occur through individual dimers and that changes between dimers in a receptor trimer or among trimer-based signaling complexes are subsequent steps in signaling.
Figures



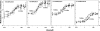

References
-
- Besschetnova, T. Y., D. J. Montefusco, A. E. Asinas, A. L. Shrout, F. M. Antommattei, and R. M. Weis. 2008. Receptor density balances signal stimulation and attenuation in membrane-assembled complexes of bacterial chemotaxis signaling proteins. Proc. Natl. Acad. Sci. U. S. A. 105:12289-12294. - PMC - PubMed
-
- Boldog, T., M. Li, and G. L. Hazelbauer. 2007. Using Nanodiscs to create water-soluble transmembrane chemoreceptors inserted in lipid bilayers. Methods Enzymol. 423:317-335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

