Characterization of a thioredoxin-thioredoxin reductase system from the hyperthermophilic bacterium Thermotoga maritima
- PMID: 20061476
- PMCID: PMC2820846
- DOI: 10.1128/JB.01035-09
Characterization of a thioredoxin-thioredoxin reductase system from the hyperthermophilic bacterium Thermotoga maritima
Abstract
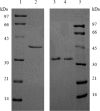
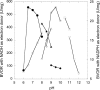
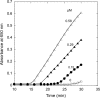
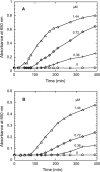
A thioredoxin reductase and a thioredoxin were purified to homogeneity from a cell extract of Thermotoga maritima. The thioredoxin reductase was a homodimeric flavin adenine dinucleotide (FAD)-containing protein with a subunit of 37 kDa estimated using SDS-PAGE, which was identified to be TM0869. The amino acid sequence of the enzyme showed high identities and similarities to those of typical bacterial thioredoxin reductases. Although the purified T. maritima thioredoxin reductase could not use thioredoxin from Spirulina as an electron acceptor, it used thioredoxin that was purified from T. maritima by monitoring the dithiothreitol-dependent reduction of bovine insulin. This enzyme also catalyzed the reduction of benzyl viologen using NADH or NADPH as an electron donor with apparent V(max) values of 1,111 +/- 35 micromol NADH oxidized min(-1)mg(-1) and 115 +/- 2.4 micromol NADPH oxidized min(-1)mg(-1), respectively. The apparent K(m) values were determined to be 89 +/- 1.1 microM, 73 +/- 1.6 microM, and 780 +/- 20 microM for benzyl viologen, NADH, and NADPH, respectively. Optimal pH values were determined to be 9.5 and 6.5 for NADH and NADPH, respectively. The enzyme activity increased along with the rise of temperature up to 95 degrees C, and more than 60% of the activity remained after incubation for 28 h at 80 degrees C. The purified T. maritima thioredoxin was a monomer with a molecular mass of 31 kDa estimated using SDS-PAGE and identified as TM0868, which exhibited both thioredoxin and thioltransferase activities. T. maritima thioredoxin and thioredoxin reductase together were able to reduce insulin or 5,5'-dithio-bis(2-nitrobenzoic acid) using NAD(P)H as an electron donor. This is the first thioredoxin-thioredoxin reductase system characterized from hyperthermophilic bacteria.
Figures
References
-
- Arnér, E. S. J., and A. Holmgren. 2000. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 267:6102-6109. - PubMed
-
- Arnér, E. S. J., J. Nordberg, and A. Holmgren. 1996. Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase. Biochem. Biophys. Res. Commun. 225:268-274. - PubMed
-
- Arnér, E. S. J., L. Zhong, A. Holmgren, and P. Lester. 1999. Preparation and assay of mammalian thioredoxin and thioredoxin reductase. Methods Enzymol. 300:226-239. - PubMed
-
- Baker, A., C. M. Payne, M. M. Briehl, and G. Powis. 1997. Thioredoxin, a gene found overexpressed in human cancer, inhibits apoptosis in vitro and in vivo. Cancer Res. 57:5162-5167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

