ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production
- PMID: 20061533
- PMCID: PMC2874479
- DOI: 10.1096/fj.09-150995
ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production
Abstract
The uptake of dietary lipids from the small intestine is a complex process that depends on the activities of specific membrane receptors with yet unknown regulatory mechanisms. Using both mouse models and human cell lines, we show here that intestinal lipid absorption by the scavenger receptor class B type 1 (SR-BI) is subject to control by retinoid signaling. Retinoic acid via retinoic acid receptors induced expression of the intestinal transcription factor ISX. ISX then repressed the expression of SR-B1 and the carotenoid-15,15'-oxygenase Bcmo1. BCMO1 acts downstream of SR-BI and converts absorbed beta,beta-carotene to the retinoic acid precursor, retinaldehyde. Using BCMO1-knockout mice, we demonstrated increased intestinal SR-BI expression and systemic beta,beta-carotene accumulation. SR-BI-dependent accumulation of beta,beta-carotene was prevented by dietary retinoids that induced ISX expression. Thus, our study revealed a diet-responsive regulatory network that controls beta,beta-carotene absorption and vitamin A production by negative feedback regulation. The role of SR-BI in the intestinal absorption of other dietary lipids, including cholesterol, fatty acids, and tocopherols, implicates retinoid signaling in the regulation of lipid absorption more generally and has clinical implications for diseases associated with dyslipidemia.
Figures
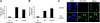
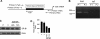
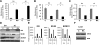
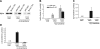
Similar articles
-
Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15'-monooxygenase (Bcmo1) expression.J Biol Chem. 2008 Feb 22;283(8):4905-11. doi: 10.1074/jbc.M707928200. Epub 2007 Dec 19. J Biol Chem. 2008. PMID: 18093975
-
Distinct pathways for the absorption and metabolism of β-carotene and zeaxanthin in the mouse intestine.J Lipid Res. 2025 Mar;66(3):100758. doi: 10.1016/j.jlr.2025.100758. Epub 2025 Feb 17. J Lipid Res. 2025. PMID: 39971162 Free PMC article.
-
Nutrigenetics of carotenoid metabolism in the chicken: a polymorphism at the β,β-carotene 15,15'-mono-oxygenase 1 (BCMO1) locus affects the response to dietary β-carotene.Br J Nutr. 2014 Jun 28;111(12):2079-88. doi: 10.1017/S0007114514000312. Epub 2014 Mar 18. Br J Nutr. 2014. PMID: 24642187
-
Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids.Biochim Biophys Acta. 2012 Jan;1821(1):70-7. doi: 10.1016/j.bbalip.2011.06.002. Epub 2011 Jun 12. Biochim Biophys Acta. 2012. PMID: 21718801 Free PMC article. Review.
-
Molecular and dietary regulation of beta,beta-carotene 15,15'-monooxygenase 1 (BCMO1).Arch Biochem Biophys. 2010 Oct 1;502(1):8-16. doi: 10.1016/j.abb.2010.06.032. Epub 2010 Jul 1. Arch Biochem Biophys. 2010. PMID: 20599666 Review.
Cited by
-
Short Communication: Oral Administration of Heat-killed Lactobacillus brevis KB290 in Combination with Retinoic Acid Provides Protection against Influenza Virus Infection in Mice.Nutrients. 2020 Sep 24;12(10):2925. doi: 10.3390/nu12102925. Nutrients. 2020. PMID: 32987850 Free PMC article.
-
The Efficacy of β-Carotene in Cow Reproduction: A Review.Animals (Basel). 2024 Jul 22;14(14):2133. doi: 10.3390/ani14142133. Animals (Basel). 2024. PMID: 39061595 Free PMC article. Review.
-
Vitamin A supply in the eye and establishment of the visual cycle.Curr Top Dev Biol. 2025;161:319-348. doi: 10.1016/bs.ctdb.2024.09.003. Epub 2024 Oct 2. Curr Top Dev Biol. 2025. PMID: 39870437 Review.
-
Genetic tuning of β-carotene oxygenase-1 activity rescues cone photoreceptor function in STRA6-deficient mice.Hum Mol Genet. 2023 Feb 19;32(5):798-809. doi: 10.1093/hmg/ddac242. Hum Mol Genet. 2023. PMID: 36150025 Free PMC article.
-
A de novo transcriptome of the noble scallop, Chlamys nobilis, focusing on mining transcripts for carotenoid-based coloration.BMC Genomics. 2015 Feb 5;16(1):44. doi: 10.1186/s12864-015-1241-x. BMC Genomics. 2015. PMID: 25651863 Free PMC article.
References
-
- Wang D Q. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–248. - PubMed
-
- Rosenblum S B, Huynh T, Afonso A, Davis H R, Jr, Yumibe N, Clader J W, Burnett D A. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4 -hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41:973–980. - PubMed
-
- Sehayek E, Nath C, Heinemann T, McGee M, Seidman C E, Samuel P, Breslow J L. U-shape relationship between change in dietary cholesterol absorption and plasma lipoprotein responsiveness and evidence for extreme interindividual variation in dietary cholesterol absorption in humans. J Lipid Res. 1998;39:2415–2422. - PubMed
-
- Borel P, Grolier P, Mekki N, Boirie Y, Rochette Y, Le Roy B, Alexandre-Gouabau M C, Lairon D, Azais-Braesco V. Low and high responders to pharmacological doses of beta-carotene: proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J Lipid Res. 1998;39:2250–2260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

