Triggering role of acid sphingomyelinase in endothelial lysosome-membrane fusion and dysfunction in coronary arteries
- PMID: 20061541
- PMCID: PMC2838547
- DOI: 10.1152/ajpheart.00958.2009
Triggering role of acid sphingomyelinase in endothelial lysosome-membrane fusion and dysfunction in coronary arteries
Abstract
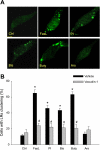
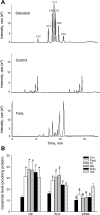
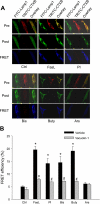
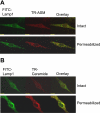
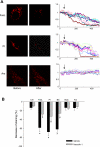
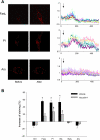
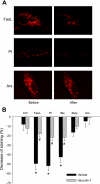
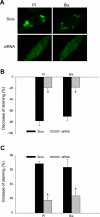
The present study determined whether activation of acid sphingomyelinase (ASM) drives membrane proximal lysosomes to fuse to the cell surface, facilitating membrane lipid rafts (LRs) clustering in coronary arterial endothelial cells (CAECs) and leading to endothelial dysfunction. By confocal microscopy, the activators of ASM, phosphatidylinositol (PI), and bis (monoacylglyceryl) phosphate (Bis), and an inducer of ASM, butyrate, were found to increase LRs clustering in bovine CAECs, which was blocked by lysosome fusion inhibitor vacuolin-1. However, arsenic trioxide (Ars), an inducer of de novo synthesis of ceramide, had no such effect. Similarly, vacuolin-1-blockable effects were observed using fluorescence resonance energy transfer detection. Liquid chromatography-electrospray ionization-tandem mass spectrometry analysis demonstrated that all of these treatments, even Ars, increased ceramide production in CAECs. When ASM gene was silenced, all treatments except Ars no longer increased ceramide levels. Furthermore, dynamic fluorescence monitoring in live cells showed that PI and Bis stimulated lysosome-membrane fusion in CAECs. Functionally, PI and Bis impaired endothelium-dependent vasodilation in perfused coronary arteries, which was blocked by vacuolin-1 and a lysosome function inhibitor, bafilomycine. FasL (Fas ligand), a previously confirmed lysosome fusion stimulator as a comparison, also produced a similar effect. It is concluded that ASM activation serves as a triggering mechanism and driving force, leading to fusion of membrane proximal lysosomes into LR clusters on the cell membrane of CAECs, which represents a novel mechanism mediating endothelial dysfunction during death receptor activation or other pathological situation.
Figures
References
-
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8: 128–140, 2007 - PubMed
-
- Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta 1746: 284–294, 2005 - PubMed
-
- Brandin L, Bergstrom G, Manhem K, Gustafsson H. Oestrogen modulates vascular adrenergic reactivity of the spontaneously hypertensive rat. J Hypertens 21: 1695–1702, 2003 - PubMed
-
- Bund SJ, Lee RM. Arterial structural changes in hypertension: a consideration of methodology, terminology and functional consequence. J Vasc Res 40: 547–557, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

