Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment
- PMID: 20061651
- PMCID: PMC2842004
- DOI: 10.3233/JAD-2010-1239
Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment
Abstract
It is now established that oxidative stress is one of the earliest, if not the earliest, change that occurs in the pathogenesis of Alzheimer's disease (AD). Consistent with this, mild cognitive impairment (MCI), the clinical precursor of AD, is also characterized by elevations in oxidative stress. Since such stress does not operate in vacuo, in this study we sought to determine whether redox-active iron, a potent source of free radicals, was elevated in MCI and preclinical AD as compared to cognitively-intact age-matched control patients. Increased iron was found at the highest levels both in the cortex and cerebellum from the pre-clinical AD/MCI cases. Interestingly, glial accumulations of redox-active iron in the cerebellum were also evident in preclinical AD patients and tended to increase as patients became progressively cognitively impaired. Our findings suggests that an imbalance in iron homeostasis is a precursor to the neurodegenerative processes leading to AD and that iron imbalance is not necessarily unique to affected regions. In fact, an understanding of iron deposition in other regions of the brain may provide insights into neuroprotective strategies. Iron deposition at the preclinical stage of AD may be useful as a diagnostic tool, using iron imaging methods, as well as a potential therapeutic target, through metal ion chelators.
Figures
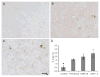
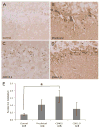

References
-
- Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. - PubMed
-
- Castellani RJ, Lee HG, Zhu X, Nunomura A, Perry G, Smith MA. Neuropathology of Alzheimer disease: pathognomonic but not pathogenic. Acta Neuropathol (Berl) 2006;111:503–509. - PubMed
-
- Hardy J. Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis. 2006;9:151–153. - PubMed
-
- Lee VM, Trojanowski JQ. Progress from Alzheimer's tangles to pathological tau points towards more effective therapies now. J Alzheimers Dis. 2006;9:257–262. - PubMed
-
- Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA. Tau phosphorylation in Alzheimer's disease: pathogen or protector? Trends Mol Med. 2005;11:164–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

