Distinct requirements for achievement of allotolerance versus reversal of autoimmunity via nonmyeloablative mixed chimerism induction in NOD mice
- PMID: 20061915
- PMCID: PMC3043373
- DOI: 10.1097/TP.0b013e3181c4692e
Distinct requirements for achievement of allotolerance versus reversal of autoimmunity via nonmyeloablative mixed chimerism induction in NOD mice
Abstract
Objectives: Mixed hematopoietic chimerism is associated with islet allograft tolerance and may reverse autoimmunity. We developed low intensity regimens for the induction of mixed chimerism and examined the effects on autoimmunity in prediabetic nonobese diabetic (NOD) mice.
Research design and methods: NOD mice received various combinations of total body irradiation, anti-CD154, anti-CD8alpha, anti-CD4, and anti-Thy1.2 monoclonal antibodies, with or without transplantation of C57BL/6 bone marrow cells and were followed up for development of diabetes, chimerism, and donor skin graft survival. Autoimmunity was assessed by histologic examination of salivary glands and pancreata.
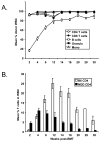
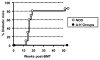
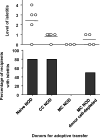
Results: Although conditioning alone prevented or delayed the onset of diabetes, stable mixed chimerism was required for the reversal of isletitis. Mixed chimerism and skin graft tolerance were achieved in NOD mice receiving anti-CD154 with bone marrow transplantation as the means of tolerizing peripheral CD4 T cells to alloantigens. However, isletitis was not reversed in allotolerant mixed chimeras prepared with this regimen.
Conclusions: Partial depletion of peripheral autoreactive NOD CD4 T cells is needed to achieve full reversal of isletitis by mixed chimerism induction from a protective donor strain, but it is not required for induction of specific tolerance to donor alloantigens. Thus, the requirements for tolerizing alloreactive and autoreactive NOD CD4 cells are distinct.
Figures
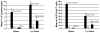



Similar articles
-
Innate and adaptive immune responses are tolerized in chimeras prepared with nonmyeloablative conditioning.Transplantation. 2012 Mar 15;93(5):469-76. doi: 10.1097/TP.0b013e318242bddf. Transplantation. 2012. PMID: 22228418 Free PMC article.
-
Development of either split tolerance or robust tolerance along with humoral tolerance to donor and third-party alloantigens in nonmyeloablative mixed chimeras.J Immunol. 2008 Apr 15;180(8):5177-86. doi: 10.4049/jimmunol.180.8.5177. J Immunol. 2008. PMID: 18390699
-
Differential outcomes in prediabetic vs. overtly diabetic NOD mice nonmyeloablatively conditioned with costimulatory blockade.Exp Hematol. 2011 Oct;39(10):977-85. doi: 10.1016/j.exphem.2011.06.008. Epub 2011 Jul 1. Exp Hematol. 2011. PMID: 21726515 Free PMC article.
-
Allogeneic hematopoietic chimerism in mice treated with sublethal myeloablation and anti-CD154 antibody: absence of graft-versus-host disease, induction of skin allograft tolerance, and prevention of recurrent autoimmunity in islet-allografted NOD/Lt mice.Blood. 2000 Mar 15;95(6):2175-82. Blood. 2000. PMID: 10706892
-
Preclinical and clinical studies on the induction of renal allograft tolerance through transient mixed chimerism.Curr Opin Organ Transplant. 2011 Aug;16(4):366-71. doi: 10.1097/MOT.0b013e3283484b2c. Curr Opin Organ Transplant. 2011. PMID: 21666482 Free PMC article. Review.
Cited by
-
Report from IPITA-TTS Opinion Leaders Meeting on the Future of β-Cell Replacement.Transplantation. 2016 Feb;100 Suppl 2(Suppl 2):S1-44. doi: 10.1097/TP.0000000000001055. Transplantation. 2016. PMID: 26840096 Free PMC article. No abstract available.
-
Curative islet and hematopoietic cell transplantation in diabetic mice without toxic bone marrow conditioning.Cell Rep. 2022 Nov 8;41(6):111615. doi: 10.1016/j.celrep.2022.111615. Cell Rep. 2022. PMID: 36351397 Free PMC article.
-
The hematopoietic system in the context of regenerative medicine.Methods. 2016 Apr 15;99:44-61. doi: 10.1016/j.ymeth.2015.08.015. Epub 2015 Aug 28. Methods. 2016. PMID: 26319943 Free PMC article. Review.
-
Mixed chimerism and split tolerance: mechanisms and clinical correlations.Chimerism. 2011 Oct-Dec;2(4):89-101. doi: 10.4161/chim.2.4.19017. Chimerism. 2011. PMID: 22509425 Free PMC article. Review.
-
Stability of Chimerism in Non-Obese Diabetic Mice Achieved By Rapid T Cell Depletion Is Associated With High Levels of Donor Cells Very Early After Transplant.Front Immunol. 2018 Apr 24;9:837. doi: 10.3389/fimmu.2018.00837. eCollection 2018. Front Immunol. 2018. PMID: 29740442 Free PMC article.
References
-
- Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. - PubMed
-
- Sykes M, Nikolic B. Treatment of severe autoimmune disease by stem-cell transplantation. Nature. 2005;435:620–627. - PubMed
-
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune diabetes through allogeneic tolerance and reversal of autoimmunity. Diabetes. 2004;53:376–383. - PubMed
-
- Saliba RM, de Lima M, Giralt S, Andersson B, Khouri IF, Hosing C, Ghosh S, Neumann J, Hsu Y, De Jesus J, Qazilbash MH, Champlin RE, Couriel DR. Hyper-acute GVHD: risk factors, outcomes, and clinical implications. Blood. 2007;109:2751–2758. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

