PPARγ controls Dectin-1 expression required for host antifungal defense against Candida albicans
- PMID: 20062524
- PMCID: PMC2795865
- DOI: 10.1371/journal.ppat.1000714
PPARγ controls Dectin-1 expression required for host antifungal defense against Candida albicans
Abstract
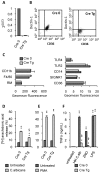
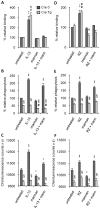
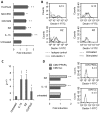
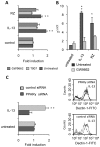
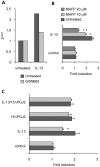
We recently showed that IL-13 or peroxisome proliferator activated receptor gamma (PPARgamma) ligands attenuate Candida albicans colonization of the gastrointestinal tract. Here, using a macrophage-specific Dectin-1 deficient mice model, we demonstrate that Dectin-1 is essential to control fungal gastrointestinal infection by PPARgamma ligands. We also show that the phagocytosis of yeast and the release of reactive oxygen intermediates in response to Candida albicans challenge are impaired in macrophages from Dectin-1 deficient mice treated with PPARgamma ligands or IL-13. Although the Mannose Receptor is not sufficient to trigger antifungal functions during the alternative activation of macrophages, our data establish the involvement of the Mannose Receptor in the initial recognition of non-opsonized Candida albicans by macrophages. We also demonstrate for the first time that the modulation of Dectin-1 expression by IL-13 involves the PPARgamma signaling pathway. These findings are consistent with a crucial role for PPARgamma in the alternative activation of macrophages by Th2 cytokines. Altogether these data suggest that PPARgamma ligands may be of therapeutic value in esophageal and gastrointestinal candidiasis in patients severely immunocompromised or with metabolic diseases in whom the prevalence of candidiasis is considerable.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008;16:27–32. - PubMed
-
- Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, et al. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–3882. - PubMed
-
- Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. - PubMed
-
- Adams EL, Rice PJ, Graves B, Ensley HE, Yu H, et al. Differential high affinity interaction of Dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side chain branching. J Pharmacol Exp Ther. 2008;325:115–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

