Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats
- PMID: 20062809
- PMCID: PMC2799530
- DOI: 10.1371/journal.pone.0008642
Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats
Abstract
Background: Pretreatment with 17beta-estradiol (E2) is profoundly neuroprotective in young animals subjected to focal and global ischemia. However, whether E2 retains its neuroprotective efficacy in aging animals, especially when administered after brain insult, is largely unknown.
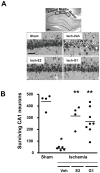
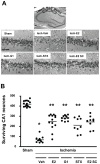
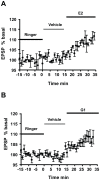
Methodology/principal findings: We examined the neuroprotective effects of E2 and two agonists that bind to non-classical estrogen receptors, G1 and STX, when administered after ischemia in middle-aged rats after prolonged ovarian hormone withdrawal. Eight weeks after ovariectomy, middle-aged female rats underwent 10 minutes of global ischemia by four vessel occlusion. Immediately after reperfusion, animals received a single infusion of either E2 (2.25 microg), G1 (50 microg) or STX (50 microg) into the lateral ventricle (ICV) or a single systemic injection of E2 (100 microg/kg). Surviving pyramidal neurons in the hippocampal CA1 were quantified 1 week later. E2 and both agonists that target non-classical estrogen receptors (G1 and STX) administered ICV at the time of reperfusion provided significant levels of neuroprotection, with 55-60% of CA1 neurons surviving vs 15% survival in controls. A single systemic injection of a pharmacological dose of E2 also rescued approximately 50% of CA1 pyramidal neurons destined to die. To determine if E2 and G1 have similar mechanisms of action in hippocampal neurons, we compared the ability of E2 and G1 to modify CA1 pyramidal neuron responses to excitatory inputs from the Schaffer collaterals recorded in hippocampal slices derived from female rats not subjected to global ischemia. E2 and G1 (10 nM) significantly potentiated pyramidal neuron responses to excitatory inputs when applied to hippocampal slices.
Conclusions/significance: These findings suggest (1) that middle-aged female rats retain their responsiveness to E2 even after a long period of hormone withdrawal, (2) that non-classical estrogen receptors may mediate the neuroprotective actions of E2 when given after ischemia, and (3) that the neuroprotective efficacy of estrogens may be related to their modulation of synaptic activity in hippocampal slices.
Conflict of interest statement
Figures

Similar articles
-
Estradiol attenuates ischemia-induced death of hippocampal neurons and enhances synaptic transmission in aged, long-term hormone-deprived female rats.PLoS One. 2012;7(6):e38018. doi: 10.1371/journal.pone.0038018. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675505 Free PMC article.
-
Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus.PLoS One. 2010 May 7;5(5):e9851. doi: 10.1371/journal.pone.0009851. PLoS One. 2010. PMID: 20479872 Free PMC article.
-
Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats.Brain Res. 2012 Sep 20;1474:82-90. doi: 10.1016/j.brainres.2012.07.025. Epub 2012 Jul 21. Brain Res. 2012. PMID: 22824334
-
Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection.Steroids. 2009 Jul;74(7):555-61. doi: 10.1016/j.steroids.2009.01.003. Epub 2009 Jan 20. Steroids. 2009. PMID: 19428444 Free PMC article. Review.
-
Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: translational implications.Front Neuroendocrinol. 2011 Aug;32(3):336-52. doi: 10.1016/j.yfrne.2010.12.005. Epub 2010 Dec 14. Front Neuroendocrinol. 2011. PMID: 21163293 Free PMC article. Review.
Cited by
-
G Protein-Coupled Estrogen Receptor: Rapid Effects on Hippocampal-Dependent Spatial Memory and Synaptic Plasticity.Front Endocrinol (Lausanne). 2020 Jun 10;11:385. doi: 10.3389/fendo.2020.00385. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32587576 Free PMC article.
-
G Protein-Coupled Estrogen Receptor GPER: Molecular Pharmacology and Therapeutic Applications.Annu Rev Pharmacol Toxicol. 2023 Jan 20;63:295-320. doi: 10.1146/annurev-pharmtox-031122-121944. Annu Rev Pharmacol Toxicol. 2023. PMID: 36662583 Free PMC article. Review.
-
The Importance of G-protein Coupled Estrogen Receptor in Patients With Fibromyalgia.Arch Rheumatol. 2019 Jan 23;34(4):419-425. doi: 10.5606/ArchRheumatol.2019.7236. eCollection 2019 Dec. Arch Rheumatol. 2019. PMID: 32010891 Free PMC article.
-
The novel estrogen receptor modulator STX attenuates Amyloid-β neurotoxicity in the 5XFAD mouse model of Alzheimer's disease.Neurobiol Dis. 2022 Nov;174:105888. doi: 10.1016/j.nbd.2022.105888. Epub 2022 Oct 6. Neurobiol Dis. 2022. PMID: 36209948 Free PMC article.
-
G protein-coupled estrogen receptor: a new therapeutic target in stroke and traumatic brain/spinal cord injury?Crit Care Med. 2012 Dec;40(12):3323-5. doi: 10.1097/CCM.0b013e31826be998. Crit Care Med. 2012. PMID: 23164781 Free PMC article. No abstract available.
References
-
- Wren BG. The benefits of oestrogen following menopause: why hormone replacement therapy should be offered to postmenopausal women. Med J Aust. 2009;190:321–325. - PubMed
-
- Wassertheil-Smoller S, Hendrix S, Limacher M, Heiss G, Kooperberg C, et al. Effect of Estrogen Plus Progestin on Stroke in Postmenopausal Women: The Women's Health Initiative: A Randomized Trial. JAMA. 2003;289:2673–2684. - PubMed
-
- Riggs BL, Hartmann LC. Selective estrogen-receptor modulators: Mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

