Identification of the Biotransformation Products of 2-Ethylhexyl 4-(N,N-Dimethylamino)benzoate
- PMID: 20062819
- PMCID: PMC2802490
- DOI: 10.1365/s10337-009-1386-3
Identification of the Biotransformation Products of 2-Ethylhexyl 4-(N,N-Dimethylamino)benzoate
Abstract
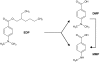
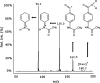
Nowadays, 2-ethylhexyl 4-(N,N-dimethylamino)benzoate (EDP) is one of the most widely used UV filters in sunscreen cosmetics and other cosmetic products. However, undesirable processes such as percutaneous absorption and biological activity have been attributed to this compound. The in vitro metabolism of EDP was elucidated in the present work. First of all, the phase I biotransformation was studied in rat liver microsomes and two metabolites, N,N-dimethyl-p-aminobenzoic acid (DMP) and N-monomethyl-p-aminobenzoic acid (MMP), were identified by GC-MS analysis. Secondly, the phase II metabolism was investigated by means of LC-MS. The investigated reactions were acetylation and glucuronidation working with rat liver cytosol and with both human and rat liver microsomes, respectively. Analogue studies with p-aminobenzoic acid (PABA) were carried out in order to compare the well established metabolic pathway of PABA with the unknown biotransformation of EDP. In addition, a method for the determination of EDP and its two phase I metabolites in human urine was developed. The methodology requires a solid-phase extraction prior to LC-MS analysis. The method is based on standard addition quantification and has been fully validated. The repeatability of the method, expressed as relative standard deviation, was in the range 3.4-7.4% and the limit of detection for all quantified analytes was in the low ng mL(-1) range.
Figures
Similar articles
-
Targeting metabolomics analysis of the sunscreen agent 2-ethylhexyl 4-(N,N-dimethylamino)benzoate in human urine by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry with liquid chromatography-time-of-flight/mass spectrometry confirmation.J Chromatogr A. 2011 May 20;1218(20):3013-21. doi: 10.1016/j.chroma.2011.03.045. Epub 2011 Mar 26. J Chromatogr A. 2011. PMID: 21481400
-
Bioaccumulation assessment of the sunscreen agent 2-ethylhexyl 4-(N,N-dimethylamino)benzoate in human semen by automated online SPE-LC-MS/MS.Anal Bioanal Chem. 2011 Aug;401(3):1003-11. doi: 10.1007/s00216-011-5141-x. Epub 2011 Jun 3. Anal Bioanal Chem. 2011. PMID: 21637928
-
The simultaneous detection and quantification of p-aminobenzoic acid and its phase 2 biotransformation metabolites in human urine using LC-MS/MS.Bioanalysis. 2015;7(10):1211-24. doi: 10.4155/bio.15.54. Bioanalysis. 2015. PMID: 26045002
-
Risk Assessment of Ethylhexyl Dimethyl PABA in Cosmetics.Toxicol Res. 2019 Apr;35(2):131-136. doi: 10.5487/TR.2019.35.2.131. Epub 2019 Apr 15. Toxicol Res. 2019. PMID: 31015895 Free PMC article. Review.
-
Safety assessment of Salicylic Acid, Butyloctyl Salicylate, Calcium Salicylate, C12-15 Alkyl Salicylate, Capryloyl Salicylic Acid, Hexyldodecyl Salicylate, Isocetyl Salicylate, Isodecyl Salicylate, Magnesium Salicylate, MEA-Salicylate, Ethylhexyl Salicylate, Potassium Salicylate, Methyl Salicylate, Myristyl Salicylate, Sodium Salicylate, TEA-Salicylate, and Tridecyl Salicylate.Int J Toxicol. 2003;22 Suppl 3:1-108. Int J Toxicol. 2003. PMID: 14617432 Review.
Cited by
-
Estimation of physicochemical properties of 2-ethylhexyl-4-methoxycinnamate (EHMC) degradation products and their toxicological evaluation.Environ Sci Pollut Res Int. 2018 Jun;25(16):16037-16049. doi: 10.1007/s11356-018-1796-6. Epub 2018 Mar 28. Environ Sci Pollut Res Int. 2018. PMID: 29594898 Free PMC article.
References
-
- Chisvert A, Salvador A (eds) (2007) Analysis of Cosmetic Products. Elsevier, Amsterdam
LinkOut - more resources
Full Text Sources
Miscellaneous
