Trityl-nitroxide biradicals as unique molecular probes for the simultaneous measurement of redox status and oxygenation
- PMID: 20062884
- PMCID: PMC4154189
- DOI: 10.1039/b919279d
Trityl-nitroxide biradicals as unique molecular probes for the simultaneous measurement of redox status and oxygenation
Abstract
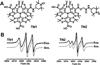
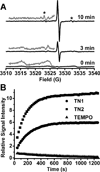
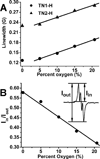
Novel trityl-nitroxide biradicals were synthesized and exhibited enhanced sensitivity and stability for rapid and simultaneous measurement of redox status and oxygenation by electron paramagnetic resonance spectroscopy.
Figures

Similar articles
-
Synthesis of trityl radical-conjugated disulfide biradicals for measurement of thiol concentration.J Org Chem. 2011 May 20;76(10):3853-60. doi: 10.1021/jo200265u. Epub 2011 Apr 29. J Org Chem. 2011. PMID: 21488696 Free PMC article.
-
Synthesis of 14N- and 15N-labeled trityl-nitroxide biradicals with strong spin-spin interaction and improved sensitivity to redox status and oxygen.J Org Chem. 2010 Nov 19;75(22):7796-802. doi: 10.1021/jo1016844. Epub 2010 Oct 28. J Org Chem. 2010. PMID: 21028905 Free PMC article.
-
Supramolecular host-guest interaction of trityl-nitroxide biradicals with cyclodextrins: modulation of spin-spin interaction and redox sensitivity.Org Biomol Chem. 2016 Feb 7;14(5):1694-701. doi: 10.1039/c5ob02450a. Org Biomol Chem. 2016. PMID: 26700002 Free PMC article.
-
Biological Relevance of Free Radicals and Nitroxides.Cell Biochem Biophys. 2017 Jun;75(2):227-240. doi: 10.1007/s12013-016-0759-0. Epub 2016 Oct 6. Cell Biochem Biophys. 2017. PMID: 27709467 Review.
-
In Vivo Molecular Electron Paramagnetic Resonance-Based Spectroscopy and Imaging of Tumor Microenvironment and Redox Using Functional Paramagnetic Probes.Antioxid Redox Signal. 2018 May 20;28(15):1365-1377. doi: 10.1089/ars.2017.7329. Epub 2017 Dec 20. Antioxid Redox Signal. 2018. PMID: 29132215 Free PMC article. Review.
Cited by
-
Structural factors controlling the spin-spin exchange coupling: EPR spectroscopic studies of highly asymmetric trityl-nitroxide biradicals.J Am Chem Soc. 2013 Feb 13;135(6):2350-6. doi: 10.1021/ja311571v. Epub 2013 Jan 30. J Am Chem Soc. 2013. PMID: 23320522 Free PMC article.
-
Tetrathiatriarylmethyl radical with a single aromatic hydrogen as a highly sensitive and specific superoxide probe.Free Radic Biol Med. 2012 Dec 1;53(11):2081-2091. doi: 10.1016/j.freeradbiomed.2012.09.011. Epub 2012 Sep 20. Free Radic Biol Med. 2012. PMID: 23000244 Free PMC article.
-
Highly bioresistant, hydrophilic and rigidly linked trityl-nitroxide biradicals for cellular high-field dynamic nuclear polarization.Chem Sci. 2022 Nov 17;13(47):14157-14164. doi: 10.1039/d2sc04668g. eCollection 2022 Dec 7. Chem Sci. 2022. PMID: 36540821 Free PMC article.
-
Efficient cross-effect dynamic nuclear polarization without depolarization in high-resolution MAS NMR.Chem Sci. 2017 Dec 1;8(12):8150-8163. doi: 10.1039/c7sc02199b. Epub 2017 Oct 2. Chem Sci. 2017. PMID: 29619170 Free PMC article.
-
Synthesis of trityl radical-conjugated disulfide biradicals for measurement of thiol concentration.J Org Chem. 2011 May 20;76(10):3853-60. doi: 10.1021/jo200265u. Epub 2011 Apr 29. J Org Chem. 2011. PMID: 21488696 Free PMC article.
References
-
- Lahti PM, editor. Magnetic Properties of Organic Materials. New York: Marcel Dekker; 1999.
- Kahn O, editor. Molecular Magnetism. VCH; Cambridge, UK: 1993.
- Miller JS, Drillon M, editors. Magnetism: Molecules to Materials. I–IV. Weinheim: Wiley-VCH; 2001–2003.
- Rajca A. Chem. Rev. 1994;94:871.
- Inoue K, Iwamura H. Angew. Chem., Int. Ed. Engl. 1995:34–927.
- Shultz DA, Farmer GT. J. Org. Chem. 1998;63:6254. - PubMed
-
- Hill NL, Braslau R. Macromolecules. 2005;38:9066.
- Lizotte JR, Anderson SG, Long TE. J. Polym. Sci., A: Polym. Chem. 2004;42:1547.
- Huang WL, Chiarelli R, Charleux B, Rassat A, Vairon JP. Macromolecules. 2002;35:2305.
-
- Marx L, Rassat A. Angew. Chem.-Int. Edit. 2000;39:4494. - PubMed
-
- Ishiguro K, Ozaki M, Sekine N, Sawaki Y. J. Am. Chem. Soc. 1997;119:3625.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

