Glucocorticoids suppress cystathionine gamma-lyase expression and H2S production in lipopolysaccharide-treated macrophages
- PMID: 20063035
- PMCID: PMC11115566
- DOI: 10.1007/s00018-009-0250-9
Glucocorticoids suppress cystathionine gamma-lyase expression and H2S production in lipopolysaccharide-treated macrophages
Abstract
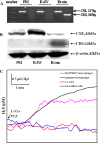
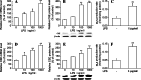
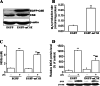
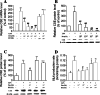
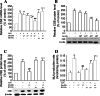
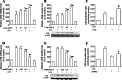
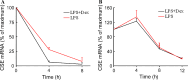
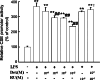
Hydrogen sulfide (H(2)S) plays an important role in inflammation. We showed that macrophages expressed the H(2)S-forming enzyme cystathionine gamma-lyase (CSE) and produced H(2)S. Lipopolysaccharide (LPS) stimulated the CSE expression and H(2)S production rate. l-cysteine reduced LPS-induced nitric oxide (NO) production. CSE inhibitor blocked the inhibitory effect of l-cysteine. CSE knockdown increased, whereas CSE overexpression decreased LPS-induced NO production. Dexamethasone suppressed LPS-induced CSE expression and the H(2)S production rate as well as NO production. l-arginine increased, whereas N(G)-nitro-l-arginine methyl ester (l-NAME) decreased LPS-induced CSE expression and H(2)S production. Dexamethasone plus l-NAME significantly decreased LPS-induced CSE expression and H(2)S production compared to l-NAME. Our results suggest that macrophages are one of the H(2)S producing sources. H(2)S might exert anti-inflammatory effects by inhibiting NO production. Dexamethasone may directly inhibit CSE expression and H(2)S production, besides the NO-dependent way. Inhibition of H(2)S and NO production may be a mechanism by which glucocorticoids coordinate the balance between pro- and anti-inflammatory mediators during inflammation.
Figures



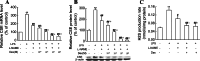
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

