The contrast dependence of the cortical fMRI deficit in amblyopia; a selective loss at higher contrasts
- PMID: 20063352
- PMCID: PMC6870632
- DOI: 10.1002/hbm.20931
The contrast dependence of the cortical fMRI deficit in amblyopia; a selective loss at higher contrasts
Abstract
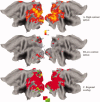
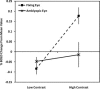
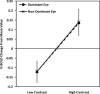
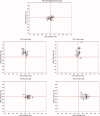
Although there is general agreement that the fMRI cortical response is reduced in humans with amblyopia, the deficit is subtle and has little correlation with threshold-based psychophysics. From a purely contrast sensitivity perspective, one would expect fMRI responses to be selectively reduced for stimuli of low contrasts. However, to date, all fMRI stimuli used in studies of amblyopia have been of high contrast. Furthermore, if the deficit is selective for low contrasts, one would expect it to reflect a selective M-cell loss, because M-cells have much higher contrast gain than P-cells and make a larger contribution to the threshold detection of stimuli of low spatial and medium temporal frequencies. To test these two predictions, we compared % BOLD response between the eyes of normals and amblyopes for low- and high-contrast stimuli using a phase-encoded design. The results suggest that the fMRI deficit in amblyopia depends upon stimulus contrast and that it is greater at high contrasts. This is consistent with a selective P-cell contrast deficit at a precortical or early cortical site.
2010 Wiley-Liss, Inc.
Figures
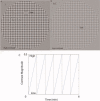
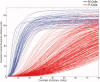
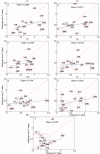


References
-
- Aaen‐Stockdale C, Hess RF ( 2008): The amblyopic deficit for global motion is spatial scale invariant. Vision Res 48: 1965–1971. - PubMed
-
- Albrecht DG, Hamilton DB ( 1982): Striate cortex of monkey and cat: Contrast response function. J Neurophysiol 48: 217–237. - PubMed
-
- Algaze A, Roberts C, Leguire L, Schmalbrock P, Rogers G ( 2002): Functional magnetic resonance imaging as a tool for investigating amblyopia in the human cortex: A pilot study. J AAPOPS 6: 300–308. - PubMed
-
- Altmann L, Singer W ( 1986): Temporal integration in amblyopic vision. Vision Res 26: 1959–1968. - PubMed
-
- Anderson SA, Holliday IE, Harding GF ( 1999): Assessment of cortical dysfunction in human strabismic amblyopia using magnetoencephalography. Vision Res 39: 1723–1738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

