A series of alpha-amino acid ester prodrugs of camptothecin: in vitro hydrolysis and A549 human lung carcinoma cell cytotoxicity
- PMID: 20063889
- PMCID: PMC3901077
- DOI: 10.1021/jm901029n
A series of alpha-amino acid ester prodrugs of camptothecin: in vitro hydrolysis and A549 human lung carcinoma cell cytotoxicity
Abstract
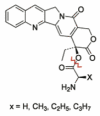
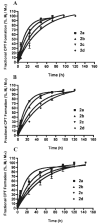
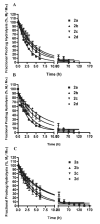
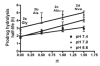
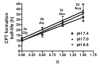
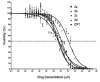
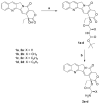
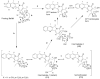
The objective of the present study was to identify a camptothecin (CPT) prodrug with optimal release and cytotoxicity properties for immobilization on a passively targeted microparticle delivery system. A series of alpha-amino acid ester prodrugs of CPT were synthesized, characterized, and evaluated. Four CPT prodrugs were synthesized with increasing aliphatic chain length (glycine (Gly) (2a), alanine (Ala) (2b), aminobutyric acid (Abu) (2c), and norvaline (Nva) (2d)). Prodrug reconversion was studied at pH 6.6, 7.0, and 7.4 corresponding to tumor, lung, and extracellular/physiological pH, respectively. Cytotoxicity was evaluated in A549 human lung carcinoma cells using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The hydrolytic reconversion rate to parent CPT increased with decreasing side chain length as well as increasing pH. The Hill slope of 2d was significantly less than CPT and the other prodrugs tested, indicating a higher cell death rate at lower concentrations. These results suggest that 2d is the best candidate for a passively targeted sustained release lung delivery system.
Figures

Similar articles
-
Supramolecular Crafting of Self-Assembling Camptothecin Prodrugs with Enhanced Efficacy against Primary Cancer Cells.Theranostics. 2016 Apr 28;6(7):1065-74. doi: 10.7150/thno.15420. eCollection 2016. Theranostics. 2016. PMID: 27217839 Free PMC article.
-
Bioreduction activated prodrugs of camptothecin: molecular design, synthesis, activation mechanism and hypoxia selective cytotoxicity.Org Biomol Chem. 2005 May 21;3(10):1905-10. doi: 10.1039/b502813b. Epub 2005 Apr 14. Org Biomol Chem. 2005. PMID: 15889173
-
Pulmonary targeting microparticulate camptothecin delivery system: anticancer evaluation in a rat orthotopic lung cancer model.Anticancer Drugs. 2010 Jan;21(1):65-76. doi: 10.1097/CAD.0b013e328332a322. Anticancer Drugs. 2010. PMID: 19966540 Free PMC article.
-
Stimulus-Responsive Nano-Prodrug Strategies for Cancer Therapy: A Focus on Camptothecin Delivery.Small Methods. 2024 Aug;8(8):e2301271. doi: 10.1002/smtd.202301271. Epub 2023 Dec 12. Small Methods. 2024. PMID: 38085682 Review.
-
Pharmacology of camptothecin esters.Ann N Y Acad Sci. 2000;922:216-23. doi: 10.1111/j.1749-6632.2000.tb07040.x. Ann N Y Acad Sci. 2000. PMID: 11193897 Review.
Cited by
-
Brain Delivery of Drug and MRI Contrast Agent: Detection and Quantitative Determination of Brain Deposition of CPT-Glu Using LC-MS/MS and Gd-DTPA Using Magnetic Resonance Imaging.Mol Pharm. 2016 Feb 1;13(2):379-90. doi: 10.1021/acs.molpharmaceut.5b00607. Epub 2016 Jan 6. Mol Pharm. 2016. PMID: 26705088 Free PMC article.
-
Tuning of the Anti-Breast Cancer Activity of Betulinic Acid via Its Conversion to Ionic Liquids.Pharmaceutics. 2024 Apr 3;16(4):496. doi: 10.3390/pharmaceutics16040496. Pharmaceutics. 2024. PMID: 38675157 Free PMC article.
-
Reactive oxygen species activated by mitochondria-specific camptothecin prodrug for enhanced chemotherapy.Bosn J Basic Med Sci. 2022 Oct 23;22(6):934-948. doi: 10.17305/bjbms.2022.7194. Bosn J Basic Med Sci. 2022. PMID: 35801419 Free PMC article.
-
C-3 alkoxymethylation of 4-oxo-1,4-dihydroquinoline 2-carboxylic acid esters via organic additives.Heliyon. 2024 May 31;10(11):e32188. doi: 10.1016/j.heliyon.2024.e32188. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38882378 Free PMC article.
-
Perspectives on biologically active camptothecin derivatives.Med Res Rev. 2015 Jul;35(4):753-89. doi: 10.1002/med.21342. Epub 2015 Mar 21. Med Res Rev. 2015. PMID: 25808858 Free PMC article. Review.
References
-
- Spataro A, Kessel D. Studies on camptothecin-induced degradation and apparent reaggregation of DNA from L1210 cells. Biochem Biophys Res Commun. 1972;48:643–648. - PubMed
-
- Li LH, Fraser TJ, Olin EJ, Bhuyan BK. Action of camptothecin on mammalian cells in culture. Cancer Res. 1972;32:2643–2650. - PubMed
-
- Horwitz SB, Chang CK, Grollman AP. Studies on camptothecin. I. Effects of nucleic acid and protein synthesis. Mol Pharmacol. 1971;7:632–644. - PubMed
-
- Hertzberg RP, Caranfa MJ, Hecht SM. On the Mechanism of Topoisomerase I Inhibition by Camptothecin: Evidence for Binding to an Enzyme-DNA Complex. Biochemistry. 1989;28:4629–4638. - PubMed
-
- Yurkovetskiy AV, Fram RJ. XMT-1001, a novel polymeric camptothecin pro-drug in clinical development for patients with advanced cancer. Adv Drug Deliv Rev. 2009 in press., doi:10.1016/j.addr.2009.01.007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

