A phase 1 study evaluating the pharmacokinetics, safety and tolerability of repeat dosing with a human IL-13 antibody (CAT-354) in subjects with asthma
- PMID: 20064211
- PMCID: PMC2820465
- DOI: 10.1186/1471-2466-10-3
A phase 1 study evaluating the pharmacokinetics, safety and tolerability of repeat dosing with a human IL-13 antibody (CAT-354) in subjects with asthma
Abstract
Background: IL-13 has been implicated in the development of airway inflammation and hyperresponsiveness. This study investigated the multiple-dose pharmacokinetics and safety profile of human anti-IL-13 antibody (CAT-354) in adults with asthma.
Methods: This was a multiple-dose, randomised, double-blind, placebo-controlled phase 1 study in asthmatics (forced expiratory volume in 1 second [FEV1] >or= 80% predicted). Subjects were randomised to receive three intravenous infusions of CAT-354 (1 mg/kg, 5 mg/kg or 10 mg/kg) or placebo at 28-day intervals. Blood samples were taken for pharmacokinetic measurements. Safety was assessed by adverse events, vital signs, ECGs, laboratory and pulmonary function parameters.
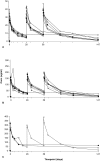
Results: Twenty-three subjects (aged 21-60 years, FEV1 88-95% predicted) received >or= 1 dose of study medication. The half-life of CAT-354 was 12-17 days and was dose-independent. The maximum serum concentration and area under the curve were dose-dependent. Clearance (2.2-2.6 mL/day/kg) and volume of distribution (44-57 mL/kg) were both low and dose-independent. The observed maximum serum concentration after each dose increased slightly from dose 1 through dose 3 at all dose levels, consistent with an accumulation ratio of 1.4 to 1.7 for area under the curve. Most adverse events were deemed mild to moderate and unrelated to study medication. One SAE was reported and deemed unrelated to study drug. There were no effects of clinical concern for vital signs, ECG, laboratory or pulmonary parameters.
Conclusions: CAT-354 exhibited linear pharmacokinetics and an acceptable safety profile. These findings suggest that at the doses tested, CAT-354 can be safely administered in multiple doses to patients with asthma.
Trial registration: NCT00974675.
Figures
References
-
- Global strategy for asthma management and prevention. 2007. http://www.ginasthma.com Last updated.
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous