Norepinephrine enhances the LPS-induced expression of COX-2 and secretion of PGE2 in primary rat microglia
- PMID: 20064241
- PMCID: PMC2819253
- DOI: 10.1186/1742-2094-7-2
Norepinephrine enhances the LPS-induced expression of COX-2 and secretion of PGE2 in primary rat microglia
Abstract
Background: Recent studies suggest an important role for neurotransmitters as modulators of inflammation. Neuroinflammatory mediators such as cytokines and molecules of the arachidonic acid pathway are generated and released by microglia. The monoamine norepinephrine reduces the production of cytokines by activated microglia in vitro. However, little is known about the effects of norepinephrine on prostanoid synthesis. In the present study, we investigate the role of norepinephrine on cyclooxygenase- (COX-)2 expression/synthesis and prostaglandin (PG)E2 production in rat primary microglia.
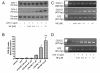
Results: Interestingly, norepinephrine increased COX-2 mRNA, but not protein expression. Norepinephrine strongly enhanced COX-2 expression and PGE2 production induced by lipopolysaccharide (LPS). This effect is likely to be mediated by beta-adrenoreceptors, since beta-, but not alpha-adrenoreceptor agonists produced similar results. Furthermore, beta-adrenoreceptor antagonists blocked the enhancement of COX-2 levels induced by norepinephrine and beta-adrenoreceptor agonists.
Conclusions: Considering that PGE2 displays different roles in neuroinflammatory and neurodegenerative disorders, norepinephrine may play an important function in the modulation of these processes in pathophysiological conditions.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

