Metabolism, cell surface organization, and disease
- PMID: 20064370
- PMCID: PMC3065826
- DOI: 10.1016/j.cell.2009.12.008
Metabolism, cell surface organization, and disease
Abstract
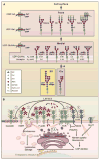
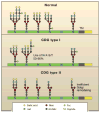
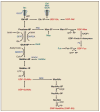
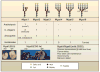
Genetic information flows from DNA to macromolecular structures-the dominant force in the molecular organization of life. However, recent work suggests that metabolite availability to the hexosamine and Golgi N-glycosylation pathways exerts control over the assembly of macromolecular complexes on the cell surface and, in this capacity, acts upstream of signaling and gene expression. The structure and number of N-glycans per protein molecule cooperate to regulate lectin binding and thereby the distribution of glycoproteins at the cell surface. Congenital disorders of glycosylation provide insight as extreme hypomorphisms, whereas milder deficiencies may encompass many common chronic conditions, including autoimmunity, metabolic syndrome, and aging.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
References
-
- Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. Journal of Biological Chemistry. 2003;279:10841–10847. - PubMed
-
- Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. - PubMed
-
- Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278:7469–7475. - PubMed
-
- Anjos S, Nguyen A, Ounissi-Benkalha H, Tessier MC, Polychronakos C. A common autoimmunity predisposing signal peptide variant of the cytotoxic T-lymphocyte antigen 4 results in inefficient glycosylation of the susceptibility allele. J Biol Chem. 2002;277:46478–46486. - PubMed
-
- Aoki M, Lin CL, Rothstein JD, Geller BA, Hosler BA, Munsat TL, Horvitz HR, Brown RH., Jr Mutations in the glutamate transporter EAAT2 gene do not cause abnormal EAAT2 transcripts in amyotrophic lateral sclerosis. Ann Neurol. 1998;43:645–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

