The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation
- PMID: 20064394
- PMCID: PMC2807410
- DOI: 10.1016/j.neuron.2009.11.007
The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation
Abstract
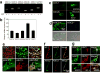
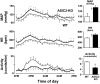
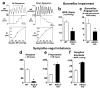
Arterial baroreceptors provide a neural sensory input that reflexly regulates the autonomic drive of circulation. Our goal was to test the hypothesis that a member of the acid-sensing ion channel (ASIC) subfamily of the DEG/ENaC superfamily is an important determinant of the arterial baroreceptor reflex. We found that aortic baroreceptor neurons in the nodose ganglia and their terminals express ASIC2. Conscious ASIC2 null mice developed hypertension, had exaggerated sympathetic and depressed parasympathetic control of the circulation, and a decreased gain of the baroreflex, all indicative of an impaired baroreceptor reflex. Multiple measures of baroreceptor activity each suggest that mechanosensitivity is diminished in ASIC2 null mice. The results define ASIC2 as an important determinant of autonomic circulatory control and of baroreceptor sensitivity. The genetic disruption of ASIC2 recapitulates the pathological dysautonomia seen in heart failure and hypertension and defines a molecular defect that may be relevant to its development.
2009 Elsevier Inc. All rights reserved.
Figures
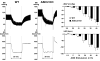



Comment in
-
A molecular sensor for the baroreceptor reflex?Neuron. 2009 Dec 24;64(6):776-7. doi: 10.1016/j.neuron.2009.12.020. Neuron. 2009. PMID: 20064383
References
-
- Landgren S. On the excitation mechanism of the carotid baroceptors. Acta Physiol Scand. 1952;26:1–34. - PubMed
-
- Heymans C, Neil E. Reflexogenic Areas of the Cardiovascular System. Boston: Little, Brown & Co; 1958. pp. 1–271.
-
- Mortara A, et al. Arterial baroreflex modulation of heart rate in chronic heart failure. Circulation. 1997;96:3450–3458. - PubMed
-
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. The Lancet. 1998;351:478–484. - PubMed
-
- Robinson TG, Dawson SL, Eames PJ, Panerai RB, Potter JF. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic stroke. Stroke. 2003;34:705–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

