Aza-tryptamine substrates in monoterpene indole alkaloid biosynthesis
- PMID: 20064432
- PMCID: PMC2807416
- DOI: 10.1016/j.chembiol.2009.11.016
Aza-tryptamine substrates in monoterpene indole alkaloid biosynthesis
Abstract
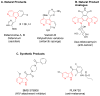
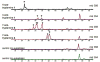
Biosynthetic pathways can be hijacked to yield novel compounds by introduction of novel starting materials. Here we have altered tryptamine, which serves as the starting substrate for a variety of alkaloid biosynthetic pathways, by replacing the indole with one of four aza-indole isomers. We show that two aza-tryptamine substrates can be successfully incorporated into the products of the monoterpene indole alkaloid pathway in Catharanthus roseus. Use of unnatural heterocycles in precursor-directed biosynthesis, in both microbial and plant natural product pathways, has not been widely demonstrated, and successful incorporation of starting substrate analogs containing the aza-indole functionality has not been previously reported. This work serves as a starting point to explore fermentation of aza-alkaloids from other tryptophan- and tryptamine-derived natural product pathways.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
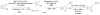
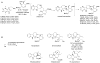
Similar articles
-
Silencing of tryptamine biosynthesis for production of nonnatural alkaloids in plant culture.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13673-8. doi: 10.1073/pnas.0903393106. Epub 2009 Aug 5. Proc Natl Acad Sci U S A. 2009. PMID: 19666570 Free PMC article.
-
Strategies for engineering plant natural products: the iridoid-derived monoterpene indole alkaloids of Catharanthus roseus.Methods Enzymol. 2012;515:189-206. doi: 10.1016/B978-0-12-394290-6.00009-4. Methods Enzymol. 2012. PMID: 22999175
-
Discovery of a Short-Chain Dehydrogenase from Catharanthus roseus that Produces a New Monoterpene Indole Alkaloid.Chembiochem. 2018 May 4;19(9):940-948. doi: 10.1002/cbic.201700621. Epub 2018 Mar 22. Chembiochem. 2018. PMID: 29424954 Free PMC article.
-
Chemistry and biology of monoterpene indole alkaloid biosynthesis.Nat Prod Rep. 2006 Aug;23(4):532-47. doi: 10.1039/b512615k. Epub 2006 May 26. Nat Prod Rep. 2006. PMID: 16874388 Review. No abstract available.
-
More than a Catharanthus plant: A multicellular and pluri-organelle alkaloid-producing factory.Curr Opin Plant Biol. 2022 Jun;67:102200. doi: 10.1016/j.pbi.2022.102200. Epub 2022 Mar 24. Curr Opin Plant Biol. 2022. PMID: 35339956 Review.
Cited by
-
Crystal structure of perakine reductase, founding member of a novel aldo-keto reductase (AKR) subfamily that undergoes unique conformational changes during NADPH binding.J Biol Chem. 2012 Mar 30;287(14):11213-21. doi: 10.1074/jbc.M111.335521. Epub 2012 Feb 13. J Biol Chem. 2012. PMID: 22334702 Free PMC article.
-
Formation of a Novel Macrocyclic Alkaloid from the Unnatural Farnesyl Diphosphate Analogue Anilinogeranyl Diphosphate by 5-Epi-Aristolochene Synthase.ACS Chem Biol. 2015 Jul 17;10(7):1729-36. doi: 10.1021/acschembio.5b00145. Epub 2015 May 4. ACS Chem Biol. 2015. PMID: 25897591 Free PMC article.
-
Unlocking New Prenylation Modes: Azaindoles as a New Substrate Class for Indole Prenyltransferases.ChemCatChem. 2023 Oct 6;15(19):e202300650. doi: 10.1002/cctc.202300650. Epub 2023 Aug 10. ChemCatChem. 2023. PMID: 37954549 Free PMC article.
-
Accelerating the semisynthesis of alkaloid-based drugs through metabolic engineering.Nat Chem Biol. 2017 Feb 15;13(3):249-258. doi: 10.1038/nchembio.2308. Nat Chem Biol. 2017. PMID: 28199297
-
Sesquiterpene Synthase-Catalysed Formation of a New Medium-Sized Cyclic Terpenoid Ether from Farnesyl Diphosphate Analogues.Chembiochem. 2018 Sep 4;19(17):1834-1838. doi: 10.1002/cbic.201800218. Epub 2018 Jul 16. Chembiochem. 2018. PMID: 29802753 Free PMC article.
References
-
- Adler TK, Albert A. The biological and physical properties of the azaindoles. J Med Chem. 1963;6:480–483. - PubMed
-
- Hemscheidt T, Zenk MH. Partial purification and characterization of a NADPH dependent tetrahydroalstonine synthase from Catharanthus roseus cell suspension cultures. Plant Cell Rep. 1985;4:216–219. - PubMed
-
- Klapars A, Buchwald SL. Copper-catalyzed halogen exchange in aryl halides: An aromatic Finkelstein reaction. J Am Chem Soc. 2002;124:14844–14845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

