Selective inhibitor of proteasome's caspase-like sites sensitizes cells to specific inhibition of chymotrypsin-like sites
- PMID: 20064438
- PMCID: PMC2807417
- DOI: 10.1016/j.chembiol.2009.11.015
Selective inhibitor of proteasome's caspase-like sites sensitizes cells to specific inhibition of chymotrypsin-like sites
Abstract
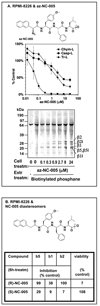
Proteasomes degrade most proteins in mammalian cells and are established targets of anticancer drugs. All eukaryotic proteasomes have three types of active sites: chymotrypsin-like, trypsin-like, and caspase-like. Chymotrypsin-like sites are the most important in protein degradation and are the primary target of most proteasome inhibitors. The biological roles of trypsin-like and caspase-like sites and their potential as cotargets of antineoplastic agents are not well defined. Here we describe the development of site-specific inhibitors and active-site probes of chymotrypsin-like and caspase-like sites. Using these compounds, we show that cytotoxicity of proteasome inhibitors does not correlate with inhibition of chymotrypsin-like sites and that coinhibition of either trypsin-like and/or caspase-like sites is needed to achieve maximal cytotoxicity. Thus, caspase-like and trypsin-like sites must be considered as cotargets of anticancer drugs.
Copyright 2009 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. - PubMed
-
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. - PubMed
-
- Altun M, Galardy PJ, Shringarpure R, Hideshima T, LeBlanc R, Anderson KC, Ploegh HL, Kessler BM. Effects of PS-341 on the activity and composition of proteasomes in multiple myeloma cells. Cancer Res. 2005;65:7896–7901. - PubMed
-
- Berkers CR, Verdoes M, Lichtman E, Fiebiger E, Kessler BM, Anderson KC, Ploegh HL, Ovaa H, Galardy PJ. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat Methods. 2005;2:357–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

