SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease
- PMID: 20064512
- PMCID: PMC2846968
- DOI: 10.1053/j.gastro.2009.12.052
SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease
Abstract
Background & aims: The appropriate alanine aminotransferase (ALT) threshold value to use for diagnosis of chronic liver disease in children is unknown. We sought to develop gender-specific, biology-based, pediatric ALT thresholds.
Methods: The Screening ALT for Elevation in Today's Youth (SAFETY) study collected observational data from acute care children's hospitals, the National Health and Nutrition Examination Survey (NHANES, 1999-2006), overweight children with and without non-alcoholic fatty liver disease (NAFLD), and children with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections. The study compared the sensitivity and specificity of ALT thresholds currently used by children's hospitals vs study-derived, gender-specific, biology-based, ALT thresholds for detecting children with NAFLD, HCV, or HBV.
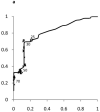
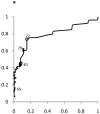
Results: The median upper limit of ALT at children's hospitals was 53 U/L (range, 30-90 U/L). The 95th percentile levels for ALT in healthy weight, metabolically normal, liver disease-free, NHANES pediatric participants were 25.8 U/L (boys) and 22.1 U/L (girls). The concordance statistics of these NHANES-derived thresholds for liver disease detection were 0.85 (95% confidence interval [CI]: 0.74-0.96) in boys and 0.91 (95% CI: 0.83-0.99) in girls for NAFLD, 0.80 (95% CI: 0.70-0.91) in boys and 0.79 (95% CI: 0.69-0.89) in girls for HBV, and 0.86 (95% CI: 0.77-0.95) in boys and 0.84 (95% CI: 0.75-0.93) in girls for HCV. Using current children's hospitals ALT thresholds, the median sensitivity for detection of NAFLD, HBV, and HCV ranged from 32% to 48%; median specificity was 92% (boys) and 96% (girls). Using NHANES-derived thresholds, the sensitivities were 72% (boys) and 82% (girls); specificities were 79% (boys) and 85% (girls).
Conclusions: The upper limit of ALT used in children's hospitals varies widely and is set too high to reliably detect chronic liver disease. Biology-based thresholds provide higher sensitivity and only slightly less specificity. Clinical guidelines for use of screening ALT and exclusion criteria for clinical trials should be modified.
Keywords: clinical trials; hepatitis; nonalcoholic fatty liver disease; obesity; patient safety.
2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Jonas MM. Children with hepatitis C. Hepatology. 2002;36:S173–178. - PubMed
-
- Barlow S, the Expert Committee Expert Committee Recommendations on the Assessment, Prevention, and Treatment of Child and Adolescent Overweight and Obesity, Summary Report. Pediatrics. 2007;120:S164–S192. - PubMed
-
- Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

