Levamisole and ryanodine receptors. I: A contraction study in Ascaris suum
- PMID: 20064566
- PMCID: PMC2838958
- DOI: 10.1016/j.molbiopara.2009.12.007
Levamisole and ryanodine receptors. I: A contraction study in Ascaris suum
Abstract
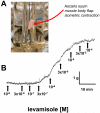
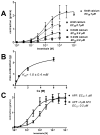
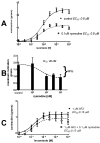
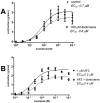
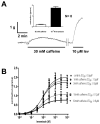
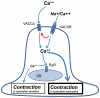
Cholinergic anthelmintics (like levamisole) are important drugs but resistance with reduced responses by the parasite to these compounds is a concern. There is a need to study and understand mechanisms that affect the amplitude of the responses of parasites to these drugs. In this paper, we study interactions of levamisole and ryanodine receptors on contractions of Ascaris suum body muscle flaps. In our second paper, we extend these observations to examine electrophysiological interactions of levamisole, ryanodine receptors (RyRs) and AF2. We report that the maximum force of contraction, g(max), was dependent on the extracellular concentration of calcium but the levamisole EC(50) (0.8 microM) was not. The relationship between maximum force of contraction and extracellular calcium was described by the Michaelis-Menten equation with a K(m) of 1.8mM. Ryanodine inhibited g(max) without effect on EC(50); ryanodine inhibited only 44% of the maximum contraction (K(i) of 40 nM), revealing a ryanodine-insensitive component in the levamisole excitation-contraction pathway. Dantrolene had the same effect as ryanodine but was less potent. The neuropeptide AF2 (1 microM) decreased the levamisole EC(50) to 0.2 microM without effect on g(max); 0.1 microM ryanodine and 100 microM dantrolene, inhibited the g(max) of the AF2-potentiated levamisole response. High concentrations of caffeine, 30 mM, produced weak contraction of the body-flap preparation. Caffeine behaved like ryanodine in that it inhibited the maximum force of contraction, g(max), without effects on the levamisole EC(50). Thus, RyRs play a modulatory role in the levamisole excitation-contraction pathway by affecting the maximum force of contraction without an effect on levamisole EC(50). The levamisole excitation-contraction coupling is graded and has at least two pathways: one sensitive to ryanodine and one not.
(c) 2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Levamisole and ryanodine receptors. II: An electrophysiological study in Ascaris suum.Mol Biochem Parasitol. 2010 May;171(1):8-16. doi: 10.1016/j.molbiopara.2009.12.006. Epub 2010 Jan 11. Mol Biochem Parasitol. 2010. PMID: 20064567 Free PMC article.
-
Brief application of AF2 produces long lasting potentiation of nAChR responses in Ascaris suum.Mol Biochem Parasitol. 2005 Jan;139(1):51-64. doi: 10.1016/j.molbiopara.2004.10.001. Mol Biochem Parasitol. 2005. PMID: 15610819
-
Effects of the muscarinic agonist, 5-methylfurmethiodide, on contraction and electrophysiology of Ascaris suum muscle.Int J Parasitol. 2008 Jul;38(8-9):945-57. doi: 10.1016/j.ijpara.2007.11.011. Epub 2007 Dec 8. Int J Parasitol. 2008. PMID: 18206155 Free PMC article.
-
Calcium release from intracellular stores and excitation-contraction coupling in intestinal smooth muscle.J Surg Res. 1997 Jul 15;71(1):79-86. doi: 10.1006/jsre.1997.5134. J Surg Res. 1997. PMID: 9271282
-
The physiology and pharmacology of neuromuscular transmission in the nematode parasite, Ascaris suum.Parasitology. 1991;102 Suppl:S41-58. doi: 10.1017/s0031182000073285. Parasitology. 1991. PMID: 1647516 Review.
Cited by
-
Expression of five acetylcholine receptor subunit genes in Brugia malayi adult worms.Int J Parasitol Drugs Drug Resist. 2015 Jun 23;5(3):100-9. doi: 10.1016/j.ijpddr.2015.04.003. eCollection 2015 Dec. Int J Parasitol Drugs Drug Resist. 2015. PMID: 26199859 Free PMC article.
-
Levamisole receptors: a second awakening.Trends Parasitol. 2012 Jul;28(7):289-96. doi: 10.1016/j.pt.2012.04.003. Epub 2012 May 17. Trends Parasitol. 2012. PMID: 22607692 Free PMC article. Review.
-
Different neuropeptides are expressed in different functional subsets of cholinergic excitatory motorneurons in the nematode Ascaris suum.ACS Chem Neurosci. 2015 Jun 17;6(6):855-70. doi: 10.1021/cn5003623. Epub 2015 Apr 9. ACS Chem Neurosci. 2015. PMID: 25812635 Free PMC article.
-
Neuronal reprograming of protein homeostasis by calcium-dependent regulation of the heat shock response.PLoS Genet. 2013 Aug;9(8):e1003711. doi: 10.1371/journal.pgen.1003711. Epub 2013 Aug 29. PLoS Genet. 2013. PMID: 24009518 Free PMC article.
-
Anthelmintic resistance and homeostatic plasticity (Brugia malayi).Sci Rep. 2021 Jul 14;11(1):14499. doi: 10.1038/s41598-021-93911-4. Sci Rep. 2021. PMID: 34262123 Free PMC article.
References
-
- Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. - PubMed
-
- Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L- and B-Subtypes of nematode nAChR Resolved at the Single-Channel Level in Ascaris suum. FASEB J. 2006;20:E2108–E2116. - PubMed
-
- Martin RJ, Robertson AP. Mode of action of levamisole and pyrantel, anthelmintic resistance, E153 and Q57. Parasitology. 2007;134:1093–1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

