Levamisole and ryanodine receptors. II: An electrophysiological study in Ascaris suum
- PMID: 20064567
- PMCID: PMC2839013
- DOI: 10.1016/j.molbiopara.2009.12.006
Levamisole and ryanodine receptors. II: An electrophysiological study in Ascaris suum
Abstract
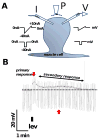
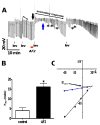
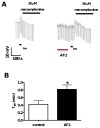
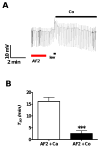
Resistance to antinematodal drugs like levamisole has increased and there is a need to understand what factors affect the responses to these anthelmintics. In our previous study, we examined the role of ryanodine receptors in muscle contraction pathways. Here we have examined interactions of levamisole receptors, ryanodine receptors (RyRs), the excitatory neuropeptide AF2, and coupling to electrophysiological responses. We examined the effects of a brief application of levamisole on Ascaris suum body muscle under current-clamp. The levamisole responses were characterized as an initial primary depolarization, followed by a slow secondary depolarizing response. We examined the effects of AF2 (KHEYLRFamide), 1 microM applied for 2 min. We found that AF2 potentiated the secondary response to levamisole and had no significant effect on the primary depolarization. Further, the reversal potentials observed during the secondary response suggested that more than one ion was involved in producing this potential. AF2 potentiated the secondary response in the presence of 30 microM mecamylamine suggesting the effect was independent of levamisole sensitive acetylcholine receptors. The secondary response, potentiated by AF2, appeared to be dependent on cytoplasmic events triggered by the primary depolarization. Ion-substitution experiments showed that the AF2 potentiated secondary response was dependent on extracellular calcium and chloride suggesting a role for the calcium-activated anion channel. Caffeine mimicked the AF2 potentiated secondary response and 0.1 microM ryanodine inhibited it. 1.0 microM ryanodine increased spiking showing that it affected membrane excitability. A model is proposed showing ryanodine receptors mediating effects of AF2 on levamisole responses.
(c) 2010 Elsevier B.V. All rights reserved.
Figures
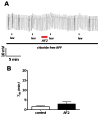
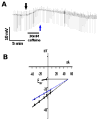


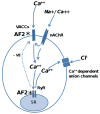
References
-
- Trailovic SM, et al. Brief application of AF2 produces long lasting potentiation of nAChR responses in Ascaris suum. Mol Biochem Parasitol. 2005;139:51–64. - PubMed
-
- Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: a pressing public health agenda for helminth control. Int J Parasitol. 2004;34:1205–10. - PubMed
-
- Albonico M, et al. Development of the egg hatch assay for detection of anthelmintic resistance in human hookworms. Int J Parasitol. 2005;35:803–11. - PubMed
-
- Jackson F. Anthelmintic resistance-the state of play. Br Vet J. 1993;149:123–38. - PubMed
-
- Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

