1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells
- PMID: 20064609
- PMCID: PMC2828517
- DOI: 10.1016/j.jsbmb.2010.01.001
1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells
Abstract
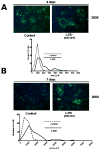
The vitamin D receptor (VDR) and its ligand 1,25D play an important role in regulating cell growth and cell fate. We examined the effect of 1,25D on cell morphology, cell proliferation, cell cycle progression and apoptosis on mesenchymal multipotent cells. Multipotent cells were treated with and without 1,25D in a time- and dose-dependent manner. Changes in cell morphology were evaluated by a green fluorescence fluorocrome. Cell proliferation was determined by the Formazan assay and PCNA antigen expression. The expression of genes related to the cell cycle was analyzed by DNA microarrays, RT(2)PCR arrays and western blots. Apoptosis was evaluated by TUNEL assay, and the expression of pro- and anti-apoptotic related genes by RT(2)PCR arrays and western blots. 1,25D inhibited cell proliferation, induced cell cycle arrest, and promoted accumulation of cells in G0/G1 phase without inducing apoptosis. An increase in cell size was associated with a decrease in the GTPase Rho and the atypical Rho family GTPase Rhou/Wrch-1 expression without inducing Wnt-1 expression. Survivin expression was also increased and may represent a novel 1,25D-mediated pathway regulating tissue injury and fibrosis. The data provide a mechanistic explanation for the anti-proliferative and anti-apoptotic properties of 1,25D in mesenchymal multipotent cells.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures





References
-
- Feldman D, Pike JW, Glorieux FH. Vitamin D. 2nd. Elsevier Academic Press; 2005. Chapter 11.
-
- Dusso AS, Brown AJ. Mechanism of vitamin D action and its regulation. Am J Kidney Dis. 1998;32:S13–S24. - PubMed
-
- Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. - PubMed
-
- Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–94. - PubMed
-
- Carlberg C, Quack M, Herdick M, Bury Y, Polly P, Toell A. Central role of VDR conformations for understanding selective actions of vitamin D(3) analogues. Steroids. 2001;66:213–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 RR019234/RR/NCRR NIH HHS/United States
- RR003026/RR/NCRR NIH HHS/United States
- U54 RR022762/RR/NCRR NIH HHS/United States
- U54RR022762/RR/NCRR NIH HHS/United States
- RR019234/RR/NCRR NIH HHS/United States
- P20 MD000182/MD/NIMHD NIH HHS/United States
- SC1NS064611/NS/NINDS NIH HHS/United States
- P20 MD000545/MD/NIMHD NIH HHS/United States
- SC1 NS064611/NS/NINDS NIH HHS/United States
- 2P20MD000182/MD/NIMHD NIH HHS/United States
- G12 RR003026/RR/NCRR NIH HHS/United States
- MD000545/MD/NIMHD NIH HHS/United States
- S21 MD000103/MD/NIMHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

