An acid-sensing ion channel from shark (Squalus acanthias) mediates transient and sustained responses to protons
- PMID: 20064854
- PMCID: PMC2834940
- DOI: 10.1113/jphysiol.2009.182931
An acid-sensing ion channel from shark (Squalus acanthias) mediates transient and sustained responses to protons
Abstract
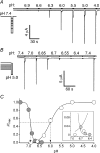
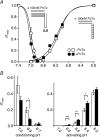
Acid-sensing ion channels (ASICs) are proton-gated Na(+) channels. They are implicated in synaptic transmission, detection of painful acidosis, and possibly sour taste. The typical ASIC current is a transient, completely desensitizing current that can be blocked by the diuretic amiloride. ASICs are present in chordates but are absent in other animals. They have been cloned from urochordates, jawless vertebrates, cartilaginous shark and bony fish, from chicken and different mammals. Strikingly, all ASICs that have so far been characterized from urochordates, jawless vertebrates and shark are not gated by protons, suggesting that proton gating evolved relatively late in bony fish and that primitive ASICs had a different and unknown gating mechanism. Recently, amino acids that are crucial for the proton gating of rat ASIC1a have been identified. These residues are completely conserved in shark ASIC1b (sASIC1b), prompting us to re-evaluate the proton sensitivity of sASIC1b. Here we show that, contrary to previous findings, sASIC1b is indeed gated by protons with half-maximal activation at pH 6.0. sASIC1b desensitizes quickly but incompletely, efficiently encoding transient as well as sustained proton signals. Our results show that the conservation of the amino acids crucial for proton gating can predict proton sensitivity of an ASIC and increase our understanding of the evolution of ASICs.
Figures
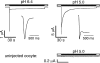





References
-
- Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem. 2001;276:35361–35367. - PubMed
-
- Bässler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Gründer S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–33787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

