Prenatal synthetic glucocorticoid exposure alters hypothalamic-pituitary-adrenal regulation and pregnancy outcomes in mature female guinea pigs
- PMID: 20064858
- PMCID: PMC2834946
- DOI: 10.1113/jphysiol.2009.182139
Prenatal synthetic glucocorticoid exposure alters hypothalamic-pituitary-adrenal regulation and pregnancy outcomes in mature female guinea pigs
Abstract
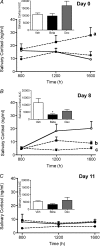
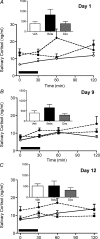
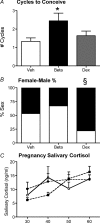
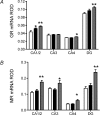
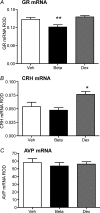
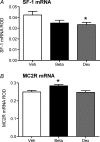
Preterm delivery occurs in approximately 10% of all pregnancies. Prenatal exposure to synthetic glucocorticoids (sGCs) reduces the incidence of respiratory distress syndrome (RDS) in these babies. Therefore, administration of multiple courses of sGCs became common practice. Animal and human studies have demonstrated that multiple courses of sGCs can have long-term effects. While the majority of animal studies have been undertaken in male offspring, it is emerging that there are profound sex differences in the consequences of prenatal sGC exposure. To our knowledge, no studies have determined the effects of prenatal sGC exposure on hypothalamic-pituitary-adrenal (HPA) axis function in female offspring while accounting for reproductive cycle status, or determined if there are effects on pregnancy parameters. Pregnant guinea pigs were administered three courses of betamethasone (Beta), dexamethasone (Dex) or vehicle on gestational days 40/41, 50/51 and 60/61. In adulthood (age range: postnatal days 126-165), basal and activated HPA axis function were assessed at various stages of the reproductive cycle. The female offspring were then mated and underwent an undisturbed pregnancy. Females were killed in the luteal phase of the reproductive cycle following litter weaning, and molecular analysis undertaken. In the luteal phase, Beta-exposed females exhibited significantly lower basal salivary cortisol levels (P < 0.05). Dex-exposed females also exhibited significantly lower basal salivary cortisol levels during the luteal phase (P < 0.05), but increased basal salivary cortisol levels during the ostrous phase (P < 0.01). The Beta-exposed females exhibited increased glucocorticoid receptor (GR) mRNA expression in the CA1/2 region of the hippocampus (P < 0.05) and MC2R mRNA in the adrenal cortex (P < 0.05). The Dex-exposed animals exhibited higher hippocampal GR and mineralocorticoid receptor (MR) mRNA levels (P < 0.05). Beta-exposed females showed reduced fecundity (P < 0.05). In Dex-exposed females there was a lower male to female sex ratio. In conclusion, prenatal sGC exposure affects HPA axis activity, in a cycle-dependent manner, and long-term reproductive success. The clinical implications of the findings on endocrine function and pregnancy in females are profound and further follow-up is warranted in human cohorts. Furthermore, we have shown there are considerable difference in phenotypes between the Beta- and Dex-exposed females and the specific endocrine and maternal outcome is contingent on the specific sGCs administered during pregnancy.
Figures
Similar articles
-
Effects of repeated prenatal glucocorticoid exposure on long-term potentiation in the juvenile guinea-pig hippocampus.J Physiol. 2007 Jun 15;581(Pt 3):1033-42. doi: 10.1113/jphysiol.2006.127381. Epub 2007 Apr 5. J Physiol. 2007. PMID: 17412773 Free PMC article.
-
Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function and blood pressure in mature male guinea pigs.J Physiol. 2004 Jul 1;558(Pt 1):305-18. doi: 10.1113/jphysiol.2004.063669. Epub 2004 May 14. J Physiol. 2004. PMID: 15146051 Free PMC article.
-
Transgenerational effects of prenatal synthetic glucocorticoids on hypothalamic-pituitary-adrenal function.Endocrinology. 2012 Jul;153(7):3295-307. doi: 10.1210/en.2012-1054. Epub 2012 May 7. Endocrinology. 2012. PMID: 22564976 Free PMC article.
-
The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset.Psychopharmacology (Berl). 2011 Mar;214(1):33-53. doi: 10.1007/s00213-010-1989-2. Epub 2010 Sep 1. Psychopharmacology (Berl). 2011. PMID: 20809212 Free PMC article. Review.
-
The Hypothalamic-Pituitary-Adrenal Axis and the Fetus.Horm Res Paediatr. 2018;89(5):380-387. doi: 10.1159/000488106. Epub 2018 Jun 6. Horm Res Paediatr. 2018. PMID: 29874660 Review.
Cited by
-
Gestational experience alters sex allocation in the subsequent generation.R Soc Open Sci. 2016 Jul 13;3(7):160210. doi: 10.1098/rsos.160210. eCollection 2016 Jul. R Soc Open Sci. 2016. PMID: 27493776 Free PMC article.
-
Testosterone is involved in mediating the effects of prenatal stress in male guinea pig offspring.J Physiol. 2011 Feb 1;589(Pt 3):755-66. doi: 10.1113/jphysiol.2010.200543. Epub 2010 Dec 20. J Physiol. 2011. PMID: 21173081 Free PMC article.
-
Assessment of in vivo fetal growth and placental vascular function in a novel intrauterine growth restriction model of progressive uterine artery occlusion in guinea pigs.J Physiol. 2016 Mar 15;594(6):1553-61. doi: 10.1113/JP271467. Epub 2016 Feb 2. J Physiol. 2016. PMID: 26719023 Free PMC article.
-
Glucocorticosteroids Effects on Brain Development in the Preterm Infant: A Role for Microglia?Curr Neuropharmacol. 2021;19(12):2188-2204. doi: 10.2174/1570159X19666210517112913. Curr Neuropharmacol. 2021. PMID: 33998994 Free PMC article. Review.
-
The proportions of term or late preterm births after exposure to early antenatal corticosteroids, and outcomes: systematic review and meta-analysis of 1.6 million infants.BMJ. 2023 Aug 2;382:e076035. doi: 10.1136/bmj-2023-076035. BMJ. 2023. PMID: 37532269 Free PMC article.
References
-
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG committee opinion no. 402: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2008;111:805–807. - PubMed
-
- Andrews MH, Matthews SG. Regulation of glucocorticoid receptor mRNA and heat shock protein 70 mRNA in the developing sheep brain. Brain Res. 2000;878:174–182. - PubMed
-
- Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–262. - PubMed
-
- Brocklehurst P, Gates S, McKenzie-McHarg K, Alfirevic Z, Chamberlain G. Are we prescribing multiple courses of antenatal corticosteroids? A survey of practice in the UK. Br J Obstet Gynaecol. 1999;106:977–979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

