Critical role of flanking residues in NGR-to-isoDGR transition and CD13/integrin receptor switching
- PMID: 20064928
- PMCID: PMC2838331
- DOI: 10.1074/jbc.M109.044297
Critical role of flanking residues in NGR-to-isoDGR transition and CD13/integrin receptor switching
Abstract
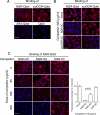
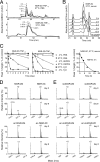
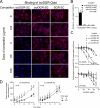
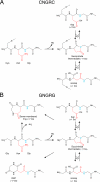
Various NGR-containing peptides have been exploited for targeted delivery of drugs to CD13-positive tumor neovasculature. Recent studies have shown that compounds containing this motif can rapidly deamidate and generate isoaspartate-glycine-arginine (isoDGR), a ligand of alphavbeta3-integrin that can be also exploited for drug delivery to tumors. We have investigated the role of NGR and isoDGR peptide scaffolds on their biochemical and biological properties. Peptides containing the cyclic CNGRC sequence could bind CD13-positive endothelial cells more efficiently than those containing linear GNGRG. Peptide degradation studies showed that cyclic peptides mostly undergo NGR-to-isoDGR transition and CD13/integrin switching, whereas linear peptides mainly undergo degradation reactions involving the alpha-amino group, which generate non-functional six/seven-membered ring compounds, unable to bind alphavbeta3, and small amount of isoDGR. Structure-activity studies showed that cyclic isoDGR could bind alphavbeta3 with an affinity >100-fold higher than that of linear isoDGR and inhibited endothelial cell adhesion and tumor growth more efficiently. Cyclic isoDGR could also bind other integrins (alphavbeta5, alphavbeta6, alphavbeta8, and alpha5beta1), although with 10-100-fold lower affinity. Peptide linearization caused loss of affinity for all integrins and loss of specificity, whereas alpha-amino group acetylation increased the affinity for all tested integrins, but caused loss of specificity. These results highlight the critical role of molecular scaffold on the biological properties of NGR/isoDGR peptides. These findings may have important implications for the design and development of anticancer drugs or tumor neovasculature-imaging compounds, and for the potential function of different NGR/isoDGR sites in natural proteins.
Figures
Similar articles
-
The neovasculature homing motif NGR: more than meets the eye.Blood. 2008 Oct 1;112(7):2628-35. doi: 10.1182/blood-2008-04-150862. Epub 2008 Jun 23. Blood. 2008. PMID: 18574027 Free PMC article. Review.
-
Systemic and tumor-targeted delivery of siRNA by cyclic NGR and isoDGR motif-containing peptides.Biomater Sci. 2016 Mar;4(3):494-510. doi: 10.1039/c5bm00429b. Biomater Sci. 2016. PMID: 26783563
-
NGR-TNF Engineering with an N-Terminal Serine Reduces Degradation and Post-Translational Modifications and Improves Its Tumor-Targeting Activity.Mol Pharm. 2020 Oct 5;17(10):3813-3824. doi: 10.1021/acs.molpharmaceut.0c00579. Epub 2020 Sep 3. Mol Pharm. 2020. PMID: 32805112
-
Structural basis for the interaction of isoDGR with the RGD-binding site of alphavbeta3 integrin.J Biol Chem. 2008 Jul 11;283(28):19757-68. doi: 10.1074/jbc.M710273200. Epub 2008 May 13. J Biol Chem. 2008. PMID: 18480047
-
Isoaspartate-dependent molecular switches for integrin-ligand recognition.J Cell Sci. 2011 Feb 15;124(Pt 4):515-22. doi: 10.1242/jcs.077172. J Cell Sci. 2011. PMID: 21282473 Review.
Cited by
-
Tumor Targeting with an isoDGR-Drug Conjugate.Chemistry. 2017 Jun 12;23(33):7910-7914. doi: 10.1002/chem.201701844. Epub 2017 May 26. Chemistry. 2017. PMID: 28449309 Free PMC article.
-
The Rational Design of Therapeutic Peptides for Aminopeptidase N using a Substrate-Based Approach.Sci Rep. 2017 May 2;7(1):1424. doi: 10.1038/s41598-017-01542-5. Sci Rep. 2017. PMID: 28465619 Free PMC article.
-
Oxidation-induced structural changes of ceruloplasmin foster NGR motif deamidation that promotes integrin binding and signaling.J Biol Chem. 2014 Feb 7;289(6):3736-48. doi: 10.1074/jbc.M113.520981. Epub 2013 Dec 23. J Biol Chem. 2014. PMID: 24366863 Free PMC article.
-
αv-Class integrin binding to fibronectin is solely mediated by RGD and unaffected by an RGE mutation.J Cell Biol. 2020 Dec 7;219(12):e202004198. doi: 10.1083/jcb.202004198. J Cell Biol. 2020. PMID: 33141174 Free PMC article.
-
Peptide-Based Strategies for Targeted Tumor Treatment and Imaging.Pharmaceutics. 2021 Apr 2;13(4):481. doi: 10.3390/pharmaceutics13040481. Pharmaceutics. 2021. PMID: 33918106 Free PMC article. Review.
References
-
- Arap W., Pasqualini R., Ruoslahti E. (1998) Science 279, 377–380 - PubMed
-
- Curnis F., Arrigoni G., Sacchi A., Fischetti L., Arap W., Pasqualini R., Corti A. (2002) Cancer Res. 62, 867–874 - PubMed
-
- Curnis F., Sacchi A., Borgna L., Magni F., Gasparri A., Corti A. (2000) Nat. Biotechnol. 18, 1185–1190 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

