P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells
- PMID: 20064929
- PMCID: PMC2844202
- DOI: 10.1074/jbc.M109.078170
P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells
Abstract
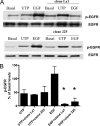
The G protein-coupled receptor P2Y(2) nucleotide receptor (P2Y(2)R) has been shown to be up-regulated in a variety of tissues in response to stress or injury. Recent studies have suggested that P2Y(2)Rs may play a role in immune responses, wound healing, and tissue regeneration via their ability to activate multiple signaling pathways, including activation of growth factor receptors. Here, we demonstrate that in human salivary gland (HSG) cells, activation of the P2Y(2)R by its agonist induces phosphorylation of ERK1/2 via two distinct mechanisms, a rapid, protein kinase C-dependent pathway and a slower and prolonged, epidermal growth factor receptor (EGFR)-dependent pathway. The EGFR-dependent stimulation of UTP-induced ERK1/2 phosphorylation in HSG cells is inhibited by the adamalysin inhibitor tumor necrosis factor-alpha protease inhibitor or by small interfering RNA that selectively silences ADAM10 and ADAM17 expression, suggesting that ADAM metalloproteases are required for P2Y(2)R-mediated activation of the EGFR. G protein-coupled receptors have been shown to promote proteolytic release of EGFR ligands; however, neutralizing antibodies to known ligands of the EGFR did not inhibit UTP-induced EGFR phosphorylation. Immunoprecipitation experiments indicated that UTP causes association of the EGFR with another member of the EGF receptor family, ErbB3. Furthermore, stimulation of HSG cells with UTP induced phosphorylation of ErbB3, and silencing of ErbB3 expression inhibited UTP-induced phosphorylation of both ErbB3 and EGFR. UTP-induced phosphorylation of ErbB3 and EGFR was also inhibited by silencing the expression of the ErbB3 ligand neuregulin 1 (NRG1). These results suggest that P2Y(2)R activation in salivary gland cells promotes the formation of EGFR/ErbB3 heterodimers and metalloprotease-dependent neuregulin 1 release, resulting in the activation of both EGFR and ErbB3.
Figures





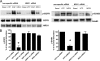

Similar articles
-
P2Y2 nucleotide receptor activation enhances the aggregation and self-organization of dispersed salivary epithelial cells.Am J Physiol Cell Physiol. 2014 Jul 1;307(1):C83-96. doi: 10.1152/ajpcell.00380.2013. Epub 2014 Apr 23. Am J Physiol Cell Physiol. 2014. PMID: 24760984 Free PMC article.
-
Cell migration in response to the amino-terminal fragment of urokinase requires epidermal growth factor receptor activation through an ADAM-mediated mechanism.J Vasc Surg. 2009 May;49(5):1296-303. doi: 10.1016/j.jvs.2008.12.026. J Vasc Surg. 2009. PMID: 19394555 Free PMC article.
-
Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors.J Biol Chem. 2004 Feb 27;279(9):8212-8. doi: 10.1074/jbc.M312230200. Epub 2003 Dec 11. J Biol Chem. 2004. PMID: 14670955
-
P2Y2 nucleotide receptor-mediated responses in brain cells.Mol Neurobiol. 2010 Jun;41(2-3):356-66. doi: 10.1007/s12035-010-8115-7. Epub 2010 Apr 13. Mol Neurobiol. 2010. PMID: 20387013 Free PMC article. Review.
-
The neuregulin-I/ErbB signaling system in development and disease.Adv Anat Embryol Cell Biol. 2007;190:1-65. Adv Anat Embryol Cell Biol. 2007. PMID: 17432114 Review.
Cited by
-
P2X7 receptor activation induces inflammatory responses in salivary gland epithelium.Am J Physiol Cell Physiol. 2012 Oct 1;303(7):C790-801. doi: 10.1152/ajpcell.00072.2012. Epub 2012 Aug 8. Am J Physiol Cell Physiol. 2012. PMID: 22875784 Free PMC article.
-
Neuroprotective roles of the P2Y(2) receptor.Purinergic Signal. 2012 Sep;8(3):559-78. doi: 10.1007/s11302-012-9307-6. Epub 2012 Apr 14. Purinergic Signal. 2012. PMID: 22528682 Free PMC article. Review.
-
P2Y2 purinergic receptor modulates virus yield, calcium homeostasis, and cell motility in human cytomegalovirus-infected cells.Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):18971-18982. doi: 10.1073/pnas.1907562116. Epub 2019 Sep 3. Proc Natl Acad Sci U S A. 2019. PMID: 31481624 Free PMC article.
-
The P2Y2 Receptor Interacts with VE-Cadherin and VEGF Receptor-2 to Regulate Rac1 Activity in Endothelial Cells.J Biomed Sci Eng. 2014 Dec 1;7(14):1105-1121. doi: 10.4236/jbise.2014.714109. J Biomed Sci Eng. 2014. PMID: 25657827 Free PMC article.
-
P2Y2 receptors mediate nucleotide-induced EGFR phosphorylation and stimulate proliferation and tumorigenesis of head and neck squamous cell carcinoma cell lines.Oral Oncol. 2020 Oct;109:104808. doi: 10.1016/j.oraloncology.2020.104808. Epub 2020 Jun 12. Oral Oncol. 2020. PMID: 32540611 Free PMC article.
References
-
- Hsieh M., Conti M. (2005) Trends Endocrinol. Metab. 16, 320–326 - PubMed
-
- Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Nat. Rev. Mol. Cell Biol. 3, 639–650 - PubMed
-
- Fukuhara S., Chikumi H., Gutkind J. S. (2001) Oncogene 20, 1661–1668 - PubMed
-
- Dhanasekaran N., Heasley L. E., Johnson G. L. (1995) Endocr. Rev. 16, 259–270 - PubMed
-
- van Biesen T., Luttrell L. M., Hawes B. E., Lefkowitz R. J. (1996) Endocr. Rev. 17, 698–714 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

