Diacylglycerol kinase delta and protein kinase C(alpha) modulate epidermal growth factor receptor abundance and degradation through ubiquitin-specific protease 8
- PMID: 20064931
- PMCID: PMC2844144
- DOI: 10.1074/jbc.M109.055731
Diacylglycerol kinase delta and protein kinase C(alpha) modulate epidermal growth factor receptor abundance and degradation through ubiquitin-specific protease 8
Abstract
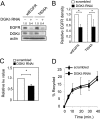
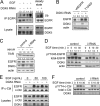
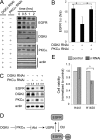
Many human epithelial cancers are characterized by abnormal activation of the epidermal growth factor receptor (EGFR), which is often caused by its excessive expression in tumor cells. The abundance of EGFR is modulated, in part, by its ubiquitination, which targets it for degradation. The components responsible for adding ubiquitin to EGFR are well characterized, but this is a reversible process, and the mechanisms that modulate the removal of ubiquitin from the EGFR are not well known. We found that de-ubiquitination of EGFR was regulated by diacylglycerol kinase delta (DGKdelta), a lipid kinase that terminates diacylglycerol signaling. In DGKdelta-deficient cells, ubiquitination of EGFR was enhanced, which attenuated the steady-state levels of EGFR and promoted its ligand-induced degradation. These effects were not caused by changes in the ubiquitinating apparatus, but instead were due to reduced expression of the de-ubiquitinase, ubiquitin-specific protease 8 (USP8). Depletion of protein kinase Calpha (PKCalpha), a target of diacylglycerol, rescued the levels of USP8 and normalized EGFR degradation in DGKdelta-deficient cells. Moreover, the effects of PKCalpha were caused by its inhibition of Akt, which stabilizes USP8. Our data indicate a novel mechanism where DGKdelta and PKCalpha modulate the levels of ubiquitinated EGFR through Akt and USP8.
Figures



Similar articles
-
Regulation of epidermal growth factor receptor ubiquitination and trafficking by the USP8·STAM complex.J Biol Chem. 2010 Nov 5;285(45):34909-21. doi: 10.1074/jbc.M109.016287. Epub 2010 Aug 24. J Biol Chem. 2010. PMID: 20736164 Free PMC article.
-
Ubiquitin-specific protease 8 is a novel prognostic marker in early-stage lung adenocarcinoma.Pathol Int. 2017 Jun;67(6):292-301. doi: 10.1111/pin.12546. Epub 2017 May 19. Pathol Int. 2017. PMID: 28544031
-
Ubiquitin-specific protease 8 links the PTEN-Akt-AIP4 pathway to the control of FLIPS stability and TRAIL sensitivity in glioblastoma multiforme.Cancer Res. 2010 Jun 15;70(12):5046-53. doi: 10.1158/0008-5472.CAN-09-3979. Epub 2010 May 18. Cancer Res. 2010. PMID: 20484045 Free PMC article.
-
USP8: a novel therapeutic target for Cushing's disease.Endocrine. 2015 Nov;50(2):292-6. doi: 10.1007/s12020-015-0682-y. Epub 2015 Jul 11. Endocrine. 2015. PMID: 26162929 Review.
-
The oncogenic role of ubiquitin specific peptidase (USP8) and its signaling pathways targeting for cancer therapeutics.Arch Biochem Biophys. 2021 Apr 15;701:108811. doi: 10.1016/j.abb.2021.108811. Epub 2021 Feb 16. Arch Biochem Biophys. 2021. PMID: 33600786 Review.
Cited by
-
Diacylglycerol kinase η modulates oncogenic properties of lung cancer cells.Clin Transl Oncol. 2014 Jan;16(1):29-35. doi: 10.1007/s12094-013-1036-y. Epub 2013 Apr 10. Clin Transl Oncol. 2014. PMID: 23572183 Free PMC article.
-
Molecular Pathways: Targeting Diacylglycerol Kinase Alpha in Cancer.Clin Cancer Res. 2015 Nov 15;21(22):5008-12. doi: 10.1158/1078-0432.CCR-15-0413. Epub 2015 Sep 29. Clin Cancer Res. 2015. PMID: 26420856 Free PMC article.
-
Identification of kinases regulating prostate cancer cell growth using an RNAi phenotypic screen.PLoS One. 2012;7(6):e38950. doi: 10.1371/journal.pone.0038950. Epub 2012 Jun 27. PLoS One. 2012. PMID: 22761715 Free PMC article.
-
Overexpression of diacylglycerol kinase η enhances Gαq-coupled G protein-coupled receptor signaling.Mol Pharmacol. 2014 May;85(5):800-10. doi: 10.1124/mol.113.091280. Epub 2014 Mar 7. Mol Pharmacol. 2014. PMID: 24608858 Free PMC article.
-
Kinectin1 depletion promotes EGFR degradation via the ubiquitin-proteosome system in cutaneous squamous cell carcinoma.Cell Death Dis. 2021 Oct 23;12(11):995. doi: 10.1038/s41419-021-04276-5. Cell Death Dis. 2021. PMID: 34689164 Free PMC article.
References
-
- Torrance C. J., Jackson P. E., Montgomery E., Kinzler K. W., Vogelstein B., Wissner A., Nunes M., Frost P., Discafani C. M. (2000) Nat. Med. 6, 1024–1028 - PubMed
-
- Buchanan F. G., Holla V., Katkuri S., Matta P., DuBois R. N. (2007) Cancer Res. 67, 9380–9388 - PubMed
-
- Sakane F., Imai S., Yamada K., Murakami T., Tsushima S., Kanoh H. (2002) J. Biol. Chem. 277, 43519–43526 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

