Reversal of Xenopus Oct25 function by disruption of the POU domain structure
- PMID: 20064932
- PMCID: PMC2832990
- DOI: 10.1074/jbc.M109.064386
Reversal of Xenopus Oct25 function by disruption of the POU domain structure
Abstract
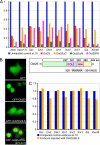
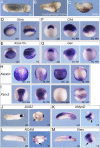
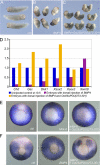
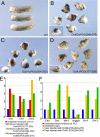
Xenopus Oct25 is a POU family subclass V (POU-V) transcription factor with a distinct domain structure. To investigate the contribution of different domains to the function of Oct25, we have performed gain of function analyses. Deletions of the N- or C-terminal regions and of the Hox domain (except its nuclear localization signal) result in mutants being indistinguishable from the wild type protein in the suppression of genes promoting germ layer formation. Deletion of the complete POU domain generates a mutant that has no effect on embryogenesis. However, disruption of the alpha-helical structures in the POU domain, even by a single amino acid mutation, causes reversal of protein function. Overexpression of such mutants leads to dorsalization of embryos and formation of secondary axial structures. The underlying mechanism is an enhanced transcription of genes coding for antagonists of the ligands for ventralizing bone morphogenetic protein and Wnt pathways. Corresponding deletion mutants of Xenopus Oct60, Oct91, or mouse Oct4 also exhibit such a dominant-negative effect. Therefore, our results reveal that the integrity of the POU domain is crucial for the function of POU-V transcription factors in the regulation of genes that promote germ layer formation.
Figures



Similar articles
-
Oct25 represses transcription of nodal/activin target genes by interaction with signal transducers during Xenopus gastrulation.J Biol Chem. 2008 Dec 5;283(49):34168-77. doi: 10.1074/jbc.M803532200. Epub 2008 Oct 15. J Biol Chem. 2008. PMID: 18922797 Free PMC article.
-
POU-V factors antagonize maternal VegT activity and beta-Catenin signaling in Xenopus embryos.EMBO J. 2007 Jun 20;26(12):2942-54. doi: 10.1038/sj.emboj.7601736. Epub 2007 May 31. EMBO J. 2007. PMID: 17541407 Free PMC article.
-
Xenopus POU factors of subclass V inhibit activin/nodal signaling during gastrulation.Mech Dev. 2006 Aug;123(8):614-25. doi: 10.1016/j.mod.2006.06.004. Epub 2006 Jun 14. Mech Dev. 2006. PMID: 16860542
-
Pou-V factor Oct25 regulates early morphogenesis in Xenopus laevis.Dev Growth Differ. 2012 Sep;54(7):702-16. doi: 10.1111/j.1440-169X.2012.01371.x. Epub 2012 Sep 7. Dev Growth Differ. 2012. PMID: 22957893
-
POU domain factors in neural development.Adv Exp Med Biol. 1998;449:39-53. doi: 10.1007/978-1-4615-4871-3_4. Adv Exp Med Biol. 1998. PMID: 10026784 Review.
Cited by
-
Regulation of germ layer formation by pluripotency factors during embryogenesis.Cell Biosci. 2013 Mar 11;3(1):15. doi: 10.1186/2045-3701-3-15. Cell Biosci. 2013. PMID: 23497659 Free PMC article.
-
Canonical WNT signaling enhances stem cell expression in the developing heart without a corresponding inhibition of cardiogenic differentiation.Stem Cells Dev. 2011 Nov;20(11):1973-83. doi: 10.1089/scd.2010.0490. Epub 2011 Apr 3. Stem Cells Dev. 2011. PMID: 21351874 Free PMC article.
References
-
- Clements D., Friday R. V., Woodland H. R. (1999) Development 126, 4903–4911 - PubMed
-
- Hyde C. E., Old R. W. (2000) Development 127, 1221–1229 - PubMed
-
- White R. J., Sun B. I., Sive H. L., Smith J. C. (2002) Development 129, 4867–4876 - PubMed
-
- Behrens J., von Kries J. P., Kühl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. (1996) Nature 382, 638–642 - PubMed
-
- Wodarz A., Nusse R. (1998) Annu. Rev. Cell Dev. Biol. 14, 59–88 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

