Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export
- PMID: 20064972
- PMCID: PMC2882738
- DOI: 10.1194/jlr.M004085
Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export
Abstract
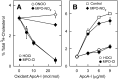
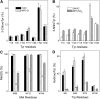
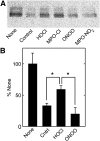
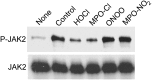
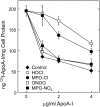
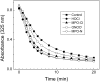
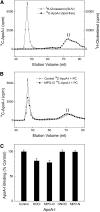
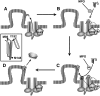
A key cardioprotective effect of high-density lipoprotein involves the interaction of its major protein, apolipoprotein A-I (apoA-I) with ATP-binding cassette transporter A1 (ABCA1), a macrophage cholesterol exporter. ApoA-I is thought to remove cholesterol from macrophages by a cascade of events. First it binds directly to ABCA1, activating signaling pathways, and then it binds to and solubilizes lipid domains generated by ABCA1. HDL isolated from human atherosclerotic lesions and blood of subjects with established coronary artery disease contains elevated levels of 3-chlorotyrosine and 3-nitrotyrosine, two characteristic products of myeloperoxidase (MPO), a heme protein secreted by macrophages. Here we show that chlorination (but not nitration) of apoA-I by the MPO pathway impairs its ability to interact directly with ABCA1, to activate the Janus kinase 2 signaling pathway, and to promote efflux of cellular cholesterol. In contrast, oxidation of apoA-I has little effect on its ability to stabilize ABCA1 protein or to solubilize phospholipids. Our results indicate that chlorination of apoA-I by the MPO pathway selectively inhibits two critical early events in cholesterol efflux: (1) the binding of apoA-I to ABCA1 and (2) the activation of a key signaling pathway. Therefore, oxidation of apoA-I in the artery wall by MPO-generated chlorinating intermediates may contribute to atherogenesis by impairing cholesterol efflux from macrophages.
Figures

Similar articles
-
Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport.J Biol Chem. 2005 Feb 18;280(7):5983-93. doi: 10.1074/jbc.M411484200. Epub 2004 Nov 30. J Biol Chem. 2005. PMID: 15574409
-
Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL.Biochim Biophys Acta. 2012 Mar;1821(3):490-501. doi: 10.1016/j.bbalip.2011.11.011. Epub 2011 Dec 10. Biochim Biophys Acta. 2012. PMID: 22178192 Free PMC article. Review.
-
Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I.J Biol Chem. 2006 Apr 7;281(14):9001-4. doi: 10.1074/jbc.C600011200. Epub 2006 Feb 22. J Biol Chem. 2006. PMID: 16497665
-
Pathways for oxidation of high-density lipoprotein in human cardiovascular disease.Curr Opin Mol Ther. 2006 Jun;8(3):198-205. Curr Opin Mol Ther. 2006. PMID: 16774039 Review.
-
Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages.J Biol Chem. 2005 Jan 7;280(1):38-47. doi: 10.1074/jbc.M407019200. Epub 2004 Oct 21. J Biol Chem. 2005. PMID: 15498770
Cited by
-
The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease.Int J Mol Sci. 2020 Jan 17;21(2):601. doi: 10.3390/ijms21020601. Int J Mol Sci. 2020. PMID: 31963445 Free PMC article. Review.
-
High-density lipoproteins, Part 1. Epidemiology, antiatherogenic effects, and therapies designed to increase their serum levels.Am J Prev Cardiol. 2025 Jul 21;23:101068. doi: 10.1016/j.ajpc.2025.101068. eCollection 2025 Sep. Am J Prev Cardiol. 2025. PMID: 40787436 Free PMC article. Review.
-
Myeloperoxidase-mediated Methionine Oxidation Promotes an Amyloidogenic Outcome for Apolipoprotein A-I.J Biol Chem. 2015 Apr 24;290(17):10958-71. doi: 10.1074/jbc.M114.630442. Epub 2015 Mar 10. J Biol Chem. 2015. PMID: 25759391 Free PMC article.
-
High-density lipoprotein, mitochondrial dysfunction and cell survival mechanisms.Chem Phys Lipids. 2016 Sep;199:161-169. doi: 10.1016/j.chemphyslip.2016.04.007. Epub 2016 May 2. Chem Phys Lipids. 2016. PMID: 27150975 Free PMC article. Review.
-
Dysfunctional High-Density Lipoproteins in Type 2 Diabetes Mellitus: Molecular Mechanisms and Therapeutic Implications.J Clin Med. 2021 May 21;10(11):2233. doi: 10.3390/jcm10112233. J Clin Med. 2021. PMID: 34063950 Free PMC article. Review.
References
-
- Kontush A., Chapman M. J. 2006. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58: 342–374. - PubMed
-
- Vaisar T., Shao B., Green P. S., Oda M. N., Oram J. F., Heinecke J. W. 2007. Myeloperoxidase and inflammatory proteins: pathways for generating dysfunctional high-density lipoprotein in humans. Curr. Atheroscler. Rep. 9: 417–424. - PubMed
-
- Leeuwenburgh C., Rasmussen J. E., Hsu F. F., Mueller D. M., Pennathur S., Heinecke J. W. 1997. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J. Biol. Chem. 272: 3520–3526. - PubMed
-
- Hurst J. K., Barrette W. C., Jr 1989. Leukocytic oxygen activation and microbicidal oxidative toxins. Crit. Rev. Biochem. Mol. Biol. 24: 271–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL055362/HL/NHLBI NIH HHS/United States
- P01 HL030086/HL/NHLBI NIH HHS/United States
- HL-078527/HL/NHLBI NIH HHS/United States
- R01 HL085437/HL/NHLBI NIH HHS/United States
- R01 HL078527/HL/NHLBI NIH HHS/United States
- K99HL091055/HL/NHLBI NIH HHS/United States
- R01 HL086798/HL/NHLBI NIH HHS/United States
- K99 HL091055/HL/NHLBI NIH HHS/United States
- HL-085437/HL/NHLBI NIH HHS/United States
- HL-030086/HL/NHLBI NIH HHS/United States
- HL-086798/HL/NHLBI NIH HHS/United States
- HL-055362/HL/NHLBI NIH HHS/United States
- R00 HL091055/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

