Intracellular trafficking of Bordetella pertussis in human macrophages
- PMID: 20065021
- PMCID: PMC2825910
- DOI: 10.1128/IAI.01031-09
Intracellular trafficking of Bordetella pertussis in human macrophages
Abstract
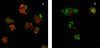
Although Bordetella pertussis has been observed to survive inside macrophages, its ability to resist or evade degradation in phagolysosomes has not been defined. We here investigated the trafficking of B. pertussis upon entry into human macrophages. During the first hours following phagocytosis, a high percentage of bacteria were destroyed within acidic compartments positive for the lysosome-associated membrane proteins (LAMP). However, roughly one-fourth of the bacteria taken up evade this initial killing event, remaining in nonacidic compartments. Forty-eight hours after infection, the number of intracellular bacteria per cell increased, suggesting that B. pertussis is capable of replicating in this type of compartment. Viable bacteria accumulated within phagosomal compartments positive for the early endosomal marker Rab5 but not the late endosomal marker LAMP. Moreover, B. pertussis-containing phagosomes acquired exogenously added transferrin, indicating that intracellular bacteria have access to extracellular components and essential nutrients via the host cell recycling pathway. Overall, these results suggest that B. pertussis survives and eventually replicates in compartments with characteristics of early endosomes, potentially contributing to its extraordinary ability to persist within hosts and populations.
Figures
Similar articles
-
Bordetella parapertussis survives inside human macrophages in lipid raft-enriched phagosomes.Infect Immun. 2014 Dec;82(12):5175-84. doi: 10.1128/IAI.02553-14. Epub 2014 Sep 29. Infect Immun. 2014. PMID: 25267839 Free PMC article.
-
Phagosome acidification has opposite effects on intracellular survival of Bordetella pertussis and B. bronchiseptica.Infect Immun. 2000 Dec;68(12):7039-48. doi: 10.1128/IAI.68.12.7039-7048.2000. Infect Immun. 2000. PMID: 11083829 Free PMC article.
-
Uptake and intracellular survival of Bordetella pertussis in human macrophages.Infect Immun. 1992 Nov;60(11):4578-85. doi: 10.1128/iai.60.11.4578-4585.1992. Infect Immun. 1992. PMID: 1398970 Free PMC article.
-
Proteome analysis of Bordetella pertussis isolated from human macrophages.J Proteomics. 2016 Mar 16;136:55-67. doi: 10.1016/j.jprot.2016.02.002. Epub 2016 Feb 10. J Proteomics. 2016. PMID: 26873878
-
Environmental sensing mechanisms in Bordetella.Adv Microb Physiol. 2001;44:141-81. doi: 10.1016/s0065-2911(01)44013-6. Adv Microb Physiol. 2001. PMID: 11407112 Review.
Cited by
-
Natural History and Ecology of Interactions Between Bordetella Species and Amoeba.Front Cell Infect Microbiol. 2022 Feb 9;12:798317. doi: 10.3389/fcimb.2022.798317. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35223538 Free PMC article.
-
Bordetella parapertussis survives inside human macrophages in lipid raft-enriched phagosomes.Infect Immun. 2014 Dec;82(12):5175-84. doi: 10.1128/IAI.02553-14. Epub 2014 Sep 29. Infect Immun. 2014. PMID: 25267839 Free PMC article.
-
Bordetella adenylate cyclase toxin differentially modulates toll-like receptor-stimulated activation, migration and T cell stimulatory capacity of dendritic cells.PLoS One. 2014 Aug 1;9(8):e104064. doi: 10.1371/journal.pone.0104064. eCollection 2014. PLoS One. 2014. PMID: 25084094 Free PMC article.
-
Characterization of a Bvg-regulated fatty acid methyl-transferase in Bordetella pertussis.PLoS One. 2017 May 11;12(5):e0176396. doi: 10.1371/journal.pone.0176396. eCollection 2017. PLoS One. 2017. PMID: 28493897 Free PMC article.
-
Conservation of Ancient Genetic Pathways for Intracellular Persistence Among Animal Pathogenic Bordetellae.Front Microbiol. 2019 Dec 11;10:2839. doi: 10.3389/fmicb.2019.02839. eCollection 2019. Front Microbiol. 2019. PMID: 31921025 Free PMC article.
References
-
- Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. - PubMed
-
- Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 97:77-89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

