CD8+ T cell concentration determines their efficiency in killing cognate antigen-expressing syngeneic mammalian cells in vitro and in mouse tissues
- PMID: 20065066
- PMCID: PMC2812553
- DOI: 10.1084/jem.20091279
CD8+ T cell concentration determines their efficiency in killing cognate antigen-expressing syngeneic mammalian cells in vitro and in mouse tissues
Abstract
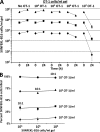
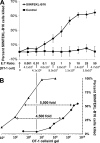
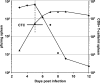
We describe a quantitative model for assessing the cytolytic activity of antigen-specific CD8+ T cells in vitro and in vivo in which the concentration of antigen-specific CD8+ T cells determines the efficiency with which these cells kill cognate antigen-expressing melanoma cells in packed cell pellets, in three-dimensional collagen-fibrin gels in vitro, and in established melanomas in vivo. In combination with a clonogenic assay for melanoma cells, collagen-fibrin gels are 4,500-5,500-fold more sensitive than the packed cell pellet-type assays generally used to measure CD8+ T cell cytolytic activity. An equation previously used to describe neutrophil bactericidal activity in vitro and in vivo also describes antigen-specific CD8+ T cell-mediated cytolysis of cognate antigen-expressing melanoma cells in collagen-fibrin gels in vitro and in transplanted tumors in vivo. We have used this equation to calculate the critical concentration of antigen-specific CD8+ T cells, which is the concentration of these cells required to hold constant the concentration of a growing population of cognate antigen-expressing melanoma cells. It is approximately 3.5x10(5)/ml collagen-fibrin gel in vitro and approximately 3x10(6)/ml or /g melanoma for previously published studies of ex vivo-activated adoptively transferred tumor antigen-specific CD8+ T cell killing of cognate antigen-expressing melanoma cells in established tumors in vivo. The antigen-specific CD8+ T cell concentration required to kill 100% of 2x10(7)/ml cognate antigen-expressing melanoma cells in collagen fibrin gels is >or=10(7)/ml of gel.
Figures





References
-
- Agger R., Petersen M.S., Petersen C.C., Hansen S.B., Stødkilde-Jørgensen H., Skands U., Blankenstein T., Andersen T.E., Hulgaard E.F., Jørgensen J.T., et al. 2007. T cell homing to tumors detected by 3D-coordinated positron emission tomography and magnetic resonance imaging. J. Immunother. 30:29–39 10.1097/01.cji.0000211326.38149.7e - DOI - PubMed
-
- Baramova E.N., Coucke P., Leprince P., De Pauw-Gillet M.C., Bassleer R., Foidart J.M. 1994. Evaluation of matrix metalloproteinases and serine proteases activities in three B16 melanoma cell lines with distinct tumorigenic potential. Anticancer Res. 14:841–846 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

