Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3
- PMID: 20065093
- PMCID: PMC2812843
- DOI: 10.1083/jcb.200903137
Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3
Abstract
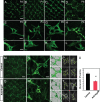
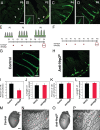
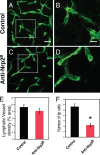
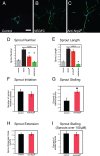
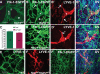
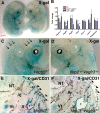
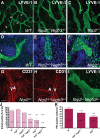
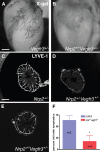
Vascular sprouting is a key process-driving development of the vascular system. In this study, we show that neuropilin-2 (Nrp2), a transmembrane receptor for the lymphangiogenic vascular endothelial growth factor C (VEGF-C), plays an important role in lymphatic vessel sprouting. Blocking VEGF-C binding to Nrp2 using antibodies specifically inhibits sprouting of developing lymphatic endothelial tip cells in vivo. In vitro analyses show that Nrp2 modulates lymphatic endothelial tip cell extension and prevents tip cell stalling and retraction during vascular sprout formation. Genetic deletion of Nrp2 reproduces the sprouting defects seen after antibody treatment. To investigate whether this defect depends on Nrp2 interaction with VEGF receptor 2 (VEGFR2) and/or 3, we intercrossed heterozygous mice lacking one allele of these receptors. Double-heterozygous nrp2vegfr2 mice develop normally without detectable lymphatic sprouting defects. In contrast, double-heterozygote nrp2vegfr3 mice show a reduction of lymphatic vessel sprouting and decreased lymph vessel branching in adult organs. Thus, interaction between Nrp2 and VEGFR3 mediates proper lymphatic vessel sprouting in response to VEGF-C.
Figures
Similar articles
-
Molecular controls of lymphatic VEGFR3 signaling.Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):421-9. doi: 10.1161/ATVBAHA.114.304881. Epub 2014 Dec 18. Arterioscler Thromb Vasc Biol. 2015. PMID: 25524775 Free PMC article.
-
Neuropilin-2 mediates lymphangiogenesis of colorectal carcinoma via a VEGFC/VEGFR3 independent signaling.Cancer Lett. 2015 Mar 28;358(2):200-209. doi: 10.1016/j.canlet.2014.12.046. Epub 2014 Dec 24. Cancer Lett. 2015. PMID: 25543087
-
Tumour necrosis factor superfamily member 15 (Tnfsf15) facilitates lymphangiogenesis via up-regulation of Vegfr3 gene expression in lymphatic endothelial cells.J Pathol. 2015 Nov;237(3):307-18. doi: 10.1002/path.4577. Epub 2015 Aug 6. J Pathol. 2015. PMID: 26096340
-
Pathway-related molecules of VEGFC/D-VEGFR3/NRP2 axis in tumor lymphangiogenesis and lymphatic metastasis.Clin Chim Acta. 2016 Oct 1;461:165-71. doi: 10.1016/j.cca.2016.08.008. Epub 2016 Aug 12. Clin Chim Acta. 2016. PMID: 27527412 Review.
-
[Morphogenesis, structure and properties of lymphatic vessels].Postepy Hig Med Dosw (Online). 2012 Nov 19;66:901-12. doi: 10.5604/17322693.1020753. Postepy Hig Med Dosw (Online). 2012. PMID: 23175346 Review. Polish.
Cited by
-
Inflammation and Lymphedema Are Exacerbated and Prolonged by Neuropilin 2 Deficiency.Am J Pathol. 2016 Nov;186(11):2803-2812. doi: 10.1016/j.ajpath.2016.07.022. Epub 2016 Oct 14. Am J Pathol. 2016. PMID: 27751443 Free PMC article.
-
A group of novel VEGF splice variants as alternative therapeutic targets in renal cell carcinoma.Mol Oncol. 2023 Jul;17(7):1379-1401. doi: 10.1002/1878-0261.13401. Epub 2023 Apr 18. Mol Oncol. 2023. PMID: 36810959 Free PMC article.
-
Targeting lymphatic vessel functions through tyrosine kinases.J Angiogenes Res. 2010 Aug 11;2:13. doi: 10.1186/2040-2384-2-13. J Angiogenes Res. 2010. PMID: 20698997 Free PMC article.
-
From lymphatic endothelial cell migration to formation of tubular lymphatic vascular network.Front Physiol. 2023 Feb 21;14:1124696. doi: 10.3389/fphys.2023.1124696. eCollection 2023. Front Physiol. 2023. PMID: 36895637 Free PMC article. Review.
-
In Vitro, In Vivo, and In Silico Models of Lymphangiogenesis in Solid Malignancies.Cancers (Basel). 2022 Mar 16;14(6):1525. doi: 10.3390/cancers14061525. Cancers (Basel). 2022. PMID: 35326676 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

