Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia
- PMID: 20065145
- PMCID: PMC2861553
- DOI: 10.1161/HYPERTENSIONAHA.109.146605
Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia
Abstract
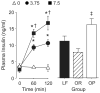
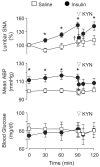
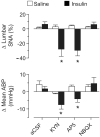
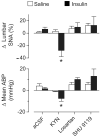
Hyperinsulinemia increases sympathetic nerve activity (SNA) and has been linked to cardiovascular morbidity in obesity. The rostral ventrolateral medulla (RVLM) plays a key role in the regulation of SNA and arterial blood pressure (ABP). Many sympathoexcitatory responses are mediated by glutamatergic receptor activation within the RVLM, and both the central renin-angiotensin and melanocortin systems are implicated in the sympathoexcitatory response to hyperinsulinemia. Therefore, we hypothesized that one or more of these neurotransmitters in the RVLM mediate the sympathoexcitatory response to insulin. Hyperinsulinemic-euglycemic clamps were performed in alpha-chloralose anesthetized, male Sprague-Dawley rats by infusion of insulin (3.75 mU/kg per minute, IV) and 50% dextrose solution for 120 minutes. Physiological increases in plasma insulin elevated lumbar SNA, with no change in renal SNA, ABP, or blood glucose. Microinjection of the ionotropic glutamate receptor antagonist kynurenic acid into the RVLM significantly reduced lumbar SNA and ABP. Selective blockade of NMDA but not non-NMDA glutamate receptors resulted in similar reductions of lumbar SNA. In marked contrast, microinjection of the angiotensin II type 1 receptor antagonist losartan or the melanocortin 3/4 antagonist SHU9119 had no effect on lumbar SNA or ABP. Western blot analysis showed that insulin receptor expression is significantly lower in the RVLM than the hypothalamus, and direct microinjection of insulin into the RVLM did not significantly increase lumbar SNA. These findings suggest that hyperinsulinemia increases lumbar SNA by activation of a glutamatergic NMDA-dependent projection to the RVLM.
Conflict of interest statement
Disclosures: None.
Figures


References
-
- Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. - PubMed
-
- Wofford MR, Hall JE. Pathophysiology and treatment of obesity hypertension. Curr Pharm Des. 2004;10:3621–3637. - PubMed
-
- Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. - PubMed
-
- Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. - PubMed
-
- Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

