Angiotensin I-converting enzyme inhibitors are allosteric enhancers of kinin B1 and B2 receptor function
- PMID: 20065150
- PMCID: PMC2814794
- DOI: 10.1161/HYPERTENSIONAHA.109.144600
Angiotensin I-converting enzyme inhibitors are allosteric enhancers of kinin B1 and B2 receptor function
Abstract
The beneficial effects of angiotensin I-converting enzyme (ACE) inhibitors go beyond the inhibition of ACE to decrease angiotensin (Ang) II or increase kinin levels. ACE inhibitors also affect kinin B1 and B2 receptor (B1R and B2R) signaling, which may underlie some of their therapeutic usefulness. They can indirectly potentiate the actions of bradykinin (BK) and ACE-resistant BK analogs on B2Rs to elevate arachidonic acid and NO release in laboratory experiments. Studies indicate that ACE inhibitors and some Ang metabolites increase B2R functions as allosteric enhancers by inducing a conformational change in ACE. This is transmitted to B2Rs via heterodimerization with ACE on the plasma membrane of cells. ACE inhibitors are also agonists of the B1R, at a Zn-binding sequence on the second extracellular loop that differs from the orthosteric binding site of the des-Arg-kinin peptide ligands. Thus, ACE inhibitors act as direct allosteric B1R agonists. When ACE inhibitors enhance B2R and B1R signaling, they augment NO production. Enhancement of B2R signaling activates endothelial NO synthase, yielding a short burst of NO; activation of B1Rs results in a prolonged high output of NO by inducible NO synthase. These actions, outside inhibiting peptide hydrolysis, may contribute to the pleiotropic therapeutic effects of ACE inhibitors in various cardiovascular disorders.
Conflict of interest statement
Figures
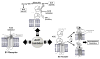
ACE inhibitors block the degradation of B2R agonist kinins, thus enhancing B2R signaling. Prolonging kinin half-life increases substrate concentration for carboxypeptidase (CP) M or N to generate des-Arg-kinins, agonists of the B1R.
Human ACE associates with B2Rs in a heterodimer on cell membranes. In the crystal structure, the N- and C-domains of ACE have a deep active site cleft and a “lid” region that restricts access of substrates or inhibitors prior to a conformational change. The two domains exhibit negative cooperativity; binding ACE inhibitor to one domain can alter the conformation of the other domain. Transmission of ACE's conformational change to the associated B2R is likely mediated by the C-domain. Enhanced mediator release or resensitization of the B2R is the consequence. (Modified from Fig. 11.3 in Erdös, EG, Tan, F and Skidgel, RA, Kinin receptors and ACE inhibitors: An interrelationship, DeMello WC, Frohlich ED, eds. Renin Angiotensin System and Cardiovascular Disease. New York: Humana Press; 2009:135-150. Used with permission of Springer Science + Business Media.)
In the third mode of action, ACE inhibitors are direct allosteric agonists of B1Rs. The canonical zinc binding motif in the second extracellular loop (HEAWH) is important for ACE inhibitors to activate B1Rs but not for des-Arg-kinin ligands. In human endothelial cells under inflammatory conditions, ACE inhibitors activate B1Rs that in turn post-translationally activate iNOS producing a prolonged high output of nitric oxide.
Similar articles
-
Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors.Neuropeptides. 2010 Apr;44(2):145-54. doi: 10.1016/j.npep.2009.12.004. Epub 2010 Jan 4. Neuropeptides. 2010. PMID: 20045558 Free PMC article. Review.
-
Antagonist, partial agonist and antiproliferative actions of B-9870 (CU201) as a function of the expression and density of the bradykinin B1 and B2 receptors.Br J Pharmacol. 2007 Feb;150(3):369-79. doi: 10.1038/sj.bjp.0706982. Epub 2006 Dec 18. Br J Pharmacol. 2007. PMID: 17179948 Free PMC article.
-
Differential regulation of collagen secretion by kinin receptors in cardiac fibroblast and myofibroblast.Toxicol Appl Pharmacol. 2012 Jun 15;261(3):300-8. doi: 10.1016/j.taap.2012.04.013. Epub 2012 Apr 18. Toxicol Appl Pharmacol. 2012. PMID: 22554775
-
[ACE inhibitors--activators of kinin receptors].Biomed Khim. 2011 May-Jun;57(3):282-99. Biomed Khim. 2011. PMID: 21863742 Review. Russian.
-
Effect of endogenous kinins, prostanoids, and NO on kinin B1 and B2 receptor expression in the rabbit.Am J Physiol. 1999 Dec;277(6):R1568-78. doi: 10.1152/ajpregu.1999.277.6.R1568. Am J Physiol. 1999. PMID: 10600901
Cited by
-
Snake venom-derived bradykinin-potentiating peptides: A promising therapy for COVID-19?Drug Dev Res. 2021 Feb;82(1):38-48. doi: 10.1002/ddr.21732. Epub 2020 Aug 5. Drug Dev Res. 2021. PMID: 32761647 Free PMC article. Review.
-
The kallikrein-kinin system and oxidative stress.Curr Opin Nephrol Hypertens. 2012 Jan;21(1):92-6. doi: 10.1097/MNH.0b013e32834d54b1. Curr Opin Nephrol Hypertens. 2012. PMID: 22048723 Free PMC article. Review.
-
Potentiation of Paclitaxel-Induced Pain Syndrome in Mice by Angiotensin I Converting Enzyme Inhibition and Involvement of Kinins.Mol Neurobiol. 2017 Dec;54(10):7824-7837. doi: 10.1007/s12035-016-0275-7. Epub 2016 Nov 14. Mol Neurobiol. 2017. PMID: 27844290
-
Kinin-kallikrein system: New perspectives in heart failure.Heart Fail Rev. 2024 May;29(3):729-737. doi: 10.1007/s10741-024-10393-y. Epub 2024 Feb 21. Heart Fail Rev. 2024. PMID: 38381277 Review.
-
Carboxypeptidase M is a positive allosteric modulator of the kinin B1 receptor.J Biol Chem. 2013 Nov 15;288(46):33226-40. doi: 10.1074/jbc.M113.520791. Epub 2013 Oct 9. J Biol Chem. 2013. PMID: 24108126 Free PMC article.
References
-
- Gavras HP, Faxon DP, Berkoben J, Brunner HR, Ryan TJ. Angiotensin converting enzyme inhibition in patients with congestive heart failure. Circulation. 1978;58:770–776. - PubMed
-
- Solomon SD, Rice MM, K AJ, Jose P, Domanski M, Sabatine M, Gersh BJ, Rouleau J, Pfeffer MA, Braunwald E. Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation. 2006;114:26–31. - PubMed
-
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. - PubMed
-
- Pfeffer MA, Frohlich ED. Improvements in clinical outcomes with the use of angiotensin-converting enzyme inhibitors: cross-fertilization between clinical and basic investigation. Am J Physiol Heart Circ Physiol. 2006;291:H2021–2025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

