Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure
- PMID: 20065166
- PMCID: PMC2823256
- DOI: 10.1161/CIRCULATIONAHA.109.882068
Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure
Abstract
Background: Cardiac cachexia is characterized by an exaggerated loss of skeletal muscle, weakness, and exercise intolerance, although the cause of these effects remains unknown. Here, we hypothesized that the heart functions as an endocrine organ in promoting systemic cachexia by secreting peptide factors such as myostatin. Myostatin is a cytokine of the transforming growth factor-beta superfamily that is known to control muscle wasting.
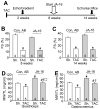
Methods and results: We used a Cre/loxP system to ablate myostatin (Mstn gene) expression in a cell type-specific manner. As expected, elimination of Mstn selectively in skeletal muscle with a myosin light chain 1f (MLC1f)-cre allele induced robust hypertrophy in all skeletal muscle. However, heart-specific deletion of Mstn with an Nkx2.5-cre allele did not alter baseline heart size or secondarily affect skeletal muscle size, but the characteristic wasting and atrophy of skeletal muscle that typify heart failure were not observed in these heart-specific null mice, indicating that myocardial myostatin expression controls muscle atrophy in heart failure. Indeed, myostatin levels in the plasma were significantly increased in wild-type mice subjected to pressure overload-induced cardiac hypertrophy but not in Mstn heart-specific deleted mice. Moreover, cardiac-specific overexpression of myostatin, which increased circulating levels of myostatin by 3- to 4-fold, caused a reduction in weight of the quadriceps, gastrocnemius, soleus, and even the heart itself. Finally, to investigate myostatin as a potential therapeutic target for the treatment of muscle wasting in heart failure, we infused a myostatin blocking antibody (JA-16), which promoted greater maintenance of muscle mass in heart failure.
Conclusions: Myostatin released from cardiomyocytes induces skeletal muscle wasting in heart failure. Targeted inhibition of myostatin in cardiac cachexia might be a therapeutic option in the future.
Figures


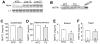
Comment in
-
Myostatin: Regulator of muscle wasting in heart failure and treatment target for cardiac cachexia.Circulation. 2010 Jan 26;121(3):354-6. doi: 10.1161/CIR.0b013e3181d0ba8b. Epub 2010 Jan 11. Circulation. 2010. PMID: 20065169 No abstract available.
References
-
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. - PubMed
-
- Lipkin DP, Jones DA, Round JM, Poole-Wilson PA. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol. 1988;18:187–195. - PubMed
-
- Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1034. - PubMed
-
- Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. - PubMed
-
- Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med. 2004;36:518–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

