Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20
- PMID: 20065188
- PMCID: PMC2849763
- DOI: 10.1200/JCO.2009.23.7610
Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20
Abstract
Purpose: The 21-gene OncotypeDX recurrence score (RS) assay quantifies the risk of distant recurrence in tamoxifen-treated patients with node-negative, estrogen receptor (ER)-positive breast cancer. We investigated the association between RS and risk for locoregional recurrence (LRR) in patients with node-negative, ER-positive breast cancer from two National Surgical Adjuvant Breast and Bowel Project (NSABP) trials (NSABP B-14 and B-20).
Patients and methods: RS was available for 895 tamoxifen-treated patients (from both trials), 355 placebo-treated patients (from B-14), and 424 chemotherapy plus tamoxifen-treated patients (from B-20). The primary end point was time to first LRR. Distant metastases, second primary cancers, and deaths before LRR were censored.
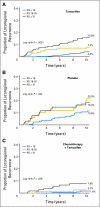
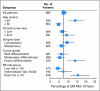
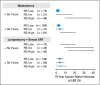
Results: In tamoxifen-treated patients, LRR was significantly associated with RS risk groups (P < .001). The 10-year Kaplan-Meier estimate of LRR was 4.% (95% CI, 2.3% to 6.3%) for patients with a low RS (< 18), 7.2% (95% CI, 3.4% to 11.0%) for those with intermediate RS (18-30), and 15.8% (95% CI, 10.4% to 21.2%) for those with a high RS (> 30). There were also significant associations between RS and LRR in placebo-treated patients from B-14 (P = .022) and in chemotherapy plus tamoxifen-treated patients from B-20 (P = .028). In multivariate analysis, RS was an independent significant predictor of LRR along with age and type of initial treatment.
Conclusion: Similar to the association between RS and risk for distant recurrence, a significant association exists between RS and risk for LRR. This information has biologic consequences and potential clinical implications relative to locoregional therapy decisions for patients with node-negative and ER-positive breast cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Defining the clinical utility of gene expression assays in breast cancer: the intersection of science and art in clinical decision making.J Clin Oncol. 2010 Apr 1;28(10):1625-7. doi: 10.1200/JCO.2009.25.2882. Epub 2010 Jan 11. J Clin Oncol. 2010. PMID: 20065178 No abstract available.
-
Molecular predictors of locoregional recurrence in breast cancer: ready for prime time?J Clin Oncol. 2010 Apr 1;28(10):1627-9. doi: 10.1200/JCO.2009.27.1080. Epub 2010 Mar 1. J Clin Oncol. 2010. PMID: 20194835 No abstract available.
-
Risk factors. Oncotype DX assay predicts local recurrence in breast cancer.Nat Rev Clin Oncol. 2010 Jun;7(6):300. doi: 10.1038/nrclinonc.2010.75. Nat Rev Clin Oncol. 2010. PMID: 20527688 No abstract available.
References
-
- Van De Vijver M, He YD, Van t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. - PubMed
-
- van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. - PubMed
-
- Foekens JA, Atkins D, Zhang Y, et al. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol. 2006;24:1665–1671. - PubMed
-
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

