Three decades of beta-lactamase inhibitors
- PMID: 20065329
- PMCID: PMC2806661
- DOI: 10.1128/CMR.00037-09
Three decades of beta-lactamase inhibitors
Abstract
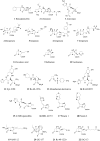
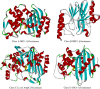
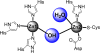
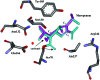
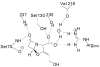
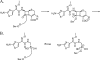
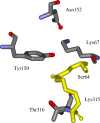
Since the introduction of penicillin, beta-lactam antibiotics have been the antimicrobial agents of choice. Unfortunately, the efficacy of these life-saving antibiotics is significantly threatened by bacterial beta-lactamases. beta-Lactamases are now responsible for resistance to penicillins, extended-spectrum cephalosporins, monobactams, and carbapenems. In order to overcome beta-lactamase-mediated resistance, beta-lactamase inhibitors (clavulanate, sulbactam, and tazobactam) were introduced into clinical practice. These inhibitors greatly enhance the efficacy of their partner beta-lactams (amoxicillin, ampicillin, piperacillin, and ticarcillin) in the treatment of serious Enterobacteriaceae and penicillin-resistant staphylococcal infections. However, selective pressure from excess antibiotic use accelerated the emergence of resistance to beta-lactam-beta-lactamase inhibitor combinations. Furthermore, the prevalence of clinically relevant beta-lactamases from other classes that are resistant to inhibition is rapidly increasing. There is an urgent need for effective inhibitors that can restore the activity of beta-lactams. Here, we review the catalytic mechanisms of each beta-lactamase class. We then discuss approaches for circumventing beta-lactamase-mediated resistance, including properties and characteristics of mechanism-based inactivators. We next highlight the mechanisms of action and salient clinical and microbiological features of beta-lactamase inhibitors. We also emphasize their therapeutic applications. We close by focusing on novel compounds and the chemical features of these agents that may contribute to a "second generation" of inhibitors. The goal for the next 3 decades will be to design inhibitors that will be effective for more than a single class of beta-lactamases.
Figures





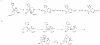
References
-
- Abraham, E. P., and E. Chain. 1940. An enzyme from bacteria able to destroy penicillin. Nature 146:837. - PubMed
-
- Abraham, E. P., E. Chain, C. M. Fletcher, H. W. Florey, A. D. Gardner, N. G. Heatley, and M. A. Jennings. 1941. Further observations on penicillin. Lancet ii:177. - PubMed
-
- Adachi, H., T. Ohta, and H. Matsuzawa. 1991. Site-directed mutants, at position 166, of RTEM-1 β-lactamase that form a stable acyl-enzyme intermediate with penicillin. J. Biol. Chem. 266:3186-3191. - PubMed
-
- Adediran, S. A., and R. F. Pratt. 2008. Inhibition of serine β-lactamases by vanadate-catechol complexes. Biochemistry 47:9467-9474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

