Industrialization of mAb production technology: the bioprocessing industry at a crossroads
- PMID: 20065641
- PMCID: PMC2759494
- DOI: 10.4161/mabs.1.5.9448
Industrialization of mAb production technology: the bioprocessing industry at a crossroads
Abstract
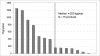
Manufacturing processes for therapeutic monoclonal antibodies (mAbs) have evolved tremendously since the first licensed mAb product in 1986. The rapid growth in product demand for mAbs triggered parallel efforts to increase production capacity through construction of large bulk manufacturing plants as well as improvements in cell culture processes to raise product titers. This combination has led to an excess of manufacturing capacity, and together with improvements in conventional purification technologies, promises nearly unlimited production capacity in the foreseeable future. The increase in titers has also led to a marked reduction in production costs, which could then become a relatively small fraction of sales price for future products which are sold at prices at or near current levels. The reduction of capacity and cost pressures for current state-of-the-art bulk production processes may shift the focus of process development efforts and have important implications for both plant design and product development strategies for both biopharmaceutical and contract manufacturing companies.
Figures

References
-
- Becker H. Muronomab-CD3 (Orthoclone OKT3) In: Dubel S, editor. Handbook of Therapeutic Antibodies Volume III: Approved Therapeutics. Weinheim: Wiley-VCH; 2007. pp. 905–940.
-
- Birch JR, Onakunle Y. Biopharmaceutical Proteins: Opportunities and Challenges. In: Smales CM, James DC, editors. Methods in Molecular Biology, vol. 308: Therapeutic Proteins: Methods and Protocols. Totowa, NJ: Humana Press Inc.; 2005. pp. 1–16. - PubMed
-
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nature. 2004;22:1393–1398. - PubMed
-
- Fahrner RL, Knudsen HL, Basey CD, Galan W, Feuerhelm D, Vanderlaan M, et al. Industrial purification of pharmaceutical antibodies: development, operation and validation of chromatography processes. Biotechnol Genet Eng Rev. 2001;18:301–327. - PubMed
-
- Shukla AA, Hubbard B, Tressel T, Gunhan S, Low D. Downstream processing of monoclonal antibodies-application of platform approaches. J Chromatogr B. 2007;848:29–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
